Analysis of αSMA-labeled progenitor cell commitment identifies notch signaling as an important pathway in fracture healing
- PMID: 24190076
- PMCID: PMC4864015
- DOI: 10.1002/jbmr.2140
Analysis of αSMA-labeled progenitor cell commitment identifies notch signaling as an important pathway in fracture healing
Abstract
Fracture healing is a regenerative process that involves coordinated responses of many cell types, but characterization of the roles of specific cell populations in this process has been limited. We have identified alpha smooth muscle actin (αSMA) as a marker of a population of mesenchymal progenitor cells in the periosteum that contributes to osteochondral elements during fracture healing. Using a lineage tracing approach, we labeled αSMA-expressing cells, and characterized changes in the periosteal population during the early stages of fracture healing by histology, flow cytometry, and gene expression profiling. In response to fracture, the αSMA-labeled population expanded and began to differentiate toward the osteogenic and chondrogenic lineages. The frequency of mesenchymal progenitor cell markers such as Sca1 and PDGFRα increased after fracture. By 6 days after fracture, genes involved in matrix production and remodeling were elevated. In contrast, genes associated with muscle contraction and Notch signaling were downregulated after fracture. We confirmed that activating Notch signaling in αSMA-labeled cells inhibited differentiation into osteogenic and adipogenic lineages in vitro and ectopic bone formation in vivo. By characterizing changes in a selected αSMA-labeled progenitor cell population during fracture callus formation, we have shown that modulation of Notch signaling may determine osteogenic potential of αSMA-expressing progenitor cells during bone healing.
Keywords: ALPHA SMOOTH MUSCLE ACTIN; FRACTURE HEALING; LINEAGE TRACING; NOTCH SIGNALING; PERIOSTEUM.
© 2014 American Society for Bone and Mineral Research.
Conflict of interest statement
All authors state that they have no conflicts of interest.
Figures


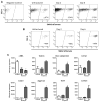
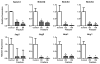

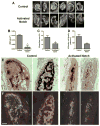
Comment in
-
Fracture repair: Could blockade of Notch signalling promote fracture repair?Nat Rev Rheumatol. 2014 Jan;10(1):3. doi: 10.1038/nrrheum.2013.186. Epub 2013 Nov 26. Nat Rev Rheumatol. 2014. PMID: 24275963 No abstract available.
References
-
- Schindeler A, McDonald MM, Bokko P, Little DG. Bone remodeling during fracture repair: the cellular picture. Semin Cell Dev Biol. 2008;19:459–66. - PubMed
-
- Ushiku C, Adams DJ, Jiang X, Wang L, Rowe DW. Long bone fracture repair in mice harboring GFP reporters for cells within the osteoblastic lineage. J Orthop Res. 2010;28:1338–47. - PubMed
-
- Olmedo ML, Landry PS, Sadasivan KK, et al. Regulation of osteoblast levels during bone healing. J Orthop Trauma. 1999;13:356–62. - PubMed
-
- Thompson Z, Miclau T, Hu D, Helms JA. A model for intramembranous ossification during fracture healing. J Orthop Res. 2002;20:1091–8. - PubMed
-
- Claes L, Recknagel S, Ignatius A. Fracture healing under healthy and inflammatory conditions. Nat Rev Rheumatol. 2012;8:133–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

