Annular PIP3 accumulation controls actin architecture and modulates cytotoxicity at the immunological synapse
- PMID: 24190432
- PMCID: PMC3832928
- DOI: 10.1084/jem.20131324
Annular PIP3 accumulation controls actin architecture and modulates cytotoxicity at the immunological synapse
Erratum in
-
Correction: Annular PIP3 accumulation controls actin architecture and modulates cytotoxicity at the immunological synapse.J Exp Med. 2017 Apr 3;214(4):1203. doi: 10.1084/jem.2013132403102017c. Epub 2017 Mar 14. J Exp Med. 2017. PMID: 28292823 Free PMC article. No abstract available.
Abstract
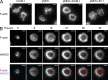
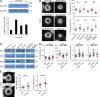
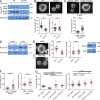
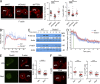
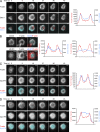
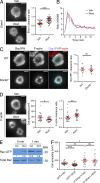
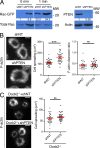
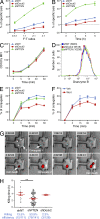
The immunological synapse formed by a T lymphocyte on the surface of a target cell contains a peripheral ring of filamentous actin (F-actin) that promotes adhesion and facilitates the directional secretion of cytokines and cytolytic factors. We show that growth and maintenance of this F-actin ring is dictated by the annular accumulation of phosphatidylinositol trisphosphate (PIP3) in the synaptic membrane. PIP3 functions in this context by recruiting the exchange factor Dock2 to the periphery of the synapse, where it drives actin polymerization through the Rho-family GTPase Rac. We also show that synaptic PIP3 is generated by class IA phosphoinositide 3-kinases that associate with T cell receptor microclusters and are activated by the GTPase Ras. Perturbations that inhibit or promote PIP3-dependent F-actin remodeling dramatically affect T cell cytotoxicity, demonstrating the functional importance of this pathway. These results reveal how T cells use lipid-based signaling to control synaptic architecture and modulate effector responses.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

