Genetic basis of neurocognitive decline and reduced white-matter integrity in normal human brain aging
- PMID: 24191011
- PMCID: PMC3839730
- DOI: 10.1073/pnas.1313735110
Genetic basis of neurocognitive decline and reduced white-matter integrity in normal human brain aging
Abstract
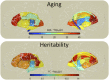
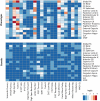
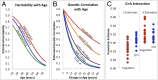
Identification of genes associated with brain aging should markedly improve our understanding of the biological processes that govern normal age-related decline. However, challenges to identifying genes that facilitate successful brain aging are considerable, including a lack of established phenotypes and difficulties in modeling the effects of aging per se, rather than genes that influence the underlying trait. In a large cohort of randomly selected pedigrees (n = 1,129 subjects), we documented profound aging effects from young adulthood to old age (18-83 y) on neurocognitive ability and diffusion-based white-matter measures. Despite significant phenotypic correlation between white-matter integrity and tests of processing speed, working memory, declarative memory, and intelligence, no evidence for pleiotropy between these classes of phenotypes was observed. Applying an advanced quantitative gene-by-environment interaction analysis where age is treated as an environmental factor, we demonstrate a heritable basis for neurocognitive deterioration as a function of age. Furthermore, by decomposing gene-by-aging (G × A) interactions, we infer that different genes influence some neurocognitive traits as a function of age, whereas other neurocognitive traits are influenced by the same genes, but to differential levels, from young adulthood to old age. In contrast, increasing white-matter incoherence with age appears to be nongenetic. These results clearly demonstrate that traits sensitive to the genetic influences on brain aging can be identified, a critical first step in delineating the biological mechanisms of successful aging.
Keywords: diffusion tensor imaging; fractional anisotropy; gene x environment interaction; genetic correlation; neurocognition.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- US Census Bureau Population Division and Housing and Household Economic Statistics Division (2008) National Population Projections (US Census Bureau, Suitland, MD)
-
- Rowe JW, Kahn RL. Human aging: Usual and successful. Science. 1987;237(4811):143–149. - PubMed
-
- Glatt SJ, Chayavichitsilp P, Depp C, Schork NJ, Jeste DV. Successful aging: From phenotype to genotype. Biol Psychiatry. 2007;62(4):282–293. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 EB015611/EB/NIBIB NIH HHS/United States
- S10 RR029392/RR/NCRR NIH HHS/United States
- MH083824/MH/NIMH NIH HHS/United States
- C06 RR017515/RR/NCRR NIH HHS/United States
- C06 RR013556/RR/NCRR NIH HHS/United States
- EB015611/EB/NIBIB NIH HHS/United States
- R01 MH059490/MH/NIMH NIH HHS/United States
- R37 MH059490/MH/NIMH NIH HHS/United States
- R01 MH083824/MH/NIMH NIH HHS/United States
- MH078111/MH/NIMH NIH HHS/United States
- MH0708143/MH/NIMH NIH HHS/United States
- R01 MH078111/MH/NIMH NIH HHS/United States
- C06 RR13556/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

