Mechanistic picture for conformational transition of a membrane transporter at atomic resolution
- PMID: 24191018
- PMCID: PMC3839739
- DOI: 10.1073/pnas.1313202110
Mechanistic picture for conformational transition of a membrane transporter at atomic resolution
Abstract
During their transport cycle, ATP-binding cassette (ABC) transporters undergo large-scale conformational changes between inward- and outward-facing states. Using an approach based on designing system-specific reaction coordinates and using nonequilibrium work relations, we have performed extensive all-atom molecular dynamics simulations in the presence of explicit membrane/solvent to sample a large number of mechanistically distinct pathways for the conformational transition of MsbA, a bacterial ABC exporter whose structure has been solved in multiple functional states. The computational approach developed here is based on (i) extensive exploration of system-specific biasing protocols (e.g., using collective variables designed based on available low-resolution crystal structures) and (ii) using nonequilibrium work relations for comparing the relevance of the transition pathways. The most relevant transition pathway identified using this approach involves several distinct stages reflecting the complex nature of the structural changes associated with the function of the protein. The opening of the cytoplasmic gate during the outward- to inward-facing transition of apo MsbA is found to be disfavored when the periplasmic gate is open and facilitated by a twisting motion of the nucleotide-binding domains that involves a dramatic change in their relative orientation. These results highlight the cooperativity between the transmembrane and the nucleotide-binding domains in the conformational transition of ABC exporters. The approach introduced here provides a framework to study large-scale conformational changes of other membrane transporters whose computational investigation at an atomic resolution may not be currently feasible using conventional methods.
Keywords: bias-exchange umbrella sampling; conformational free energy; orientation quaternion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

 is colored yellow/green, and TMD bundles B1 (
is colored yellow/green, and TMD bundles B1 ( ,
, helices), B2 (
helices), B2 ( ,
, ), B3
), B3
 , and B4
, and B4
 are colored blue, red, yellow, and green, respectively. In the OF conformation, the roll axes of bundles B1/B4 and B2/B3 are colored blue and red, respectively, to illustrate the definition of β. In the IF-o conformation, the roll axes of bundles B1/B3 and B2/B4 are colored blue and red, respectively, to illustrate the definition of α. The roll axes of
are colored blue, red, yellow, and green, respectively. In the OF conformation, the roll axes of bundles B1/B4 and B2/B3 are colored blue and red, respectively, to illustrate the definition of β. In the IF-o conformation, the roll axes of bundles B1/B3 and B2/B4 are colored blue and red, respectively, to illustrate the definition of α. The roll axes of  in the OF and IF-c conformations are colored yellow/green to illustrate the definition of γ. (B) Side (Top and Middle) and top (Bottom) views of the NBDs in OF and IF-c conformations along with the definitions of
in the OF and IF-c conformations are colored yellow/green to illustrate the definition of γ. (B) Side (Top and Middle) and top (Bottom) views of the NBDs in OF and IF-c conformations along with the definitions of  (Top) and γ (Middle).
(Top) and γ (Middle).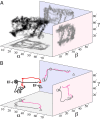
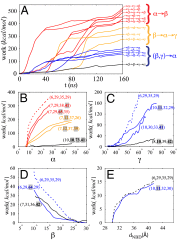
 spaces. Dashed lines are those starting from the OF conformation; (α, β, γ, dNBD) vectors show the initial state of each simulation; highlighted are the reaction coordinates with a major change before the simulation.
spaces. Dashed lines are those starting from the OF conformation; (α, β, γ, dNBD) vectors show the initial state of each simulation; highlighted are the reaction coordinates with a major change before the simulation.
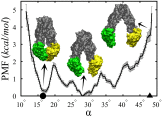
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

