MCPIP1 restricts HIV infection and is rapidly degraded in activated CD4+ T cells
- PMID: 24191027
- PMCID: PMC3839757
- DOI: 10.1073/pnas.1316208110
MCPIP1 restricts HIV infection and is rapidly degraded in activated CD4+ T cells
Abstract
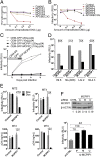
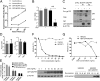
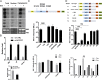
HIV-1 primarily infects activated CD4+ T cells and macrophages. Quiescent CD4+ T cells, however, possess cellular factors that limit HIV-1 infection at different postentry steps of the viral life cycle. Here, we show that the previously reported immune regulator monocyte chemotactic protein-induced protein 1 (MCPIP1) restricts HIV-1 production in CD4+ T cells. While the ectopic expression of MCPIP1 in cell lines abolished the production of HIV-1, silencing of MCPIP1 enhanced HIV-1 production. Subsequent analysis indicated that MCPIP1 imposes its restriction by decreasing the steady levels of viral mRNA species through its RNase domain. Remarkably, common T-cell stimuli induced the rapid degradation of MCPIP1 in both T-cell lines and quiescent human CD4+ T cells. Lastly, blocking the proteosomal degradation of MCPIP1 by MG132 abrogated HIV-1 production in phorbol 12-myristate 13-acetate/ionomycin-stimulated human CD4+ T cells isolated from healthy donors. Overall, MCPIP1 poses a potent barrier against HIV-1 infection at a posttranscriptional stage. Although the observed HIV restriction conferred by MCPIP1 does not seem to be overcome by any viral protein, it is removed during cellular stimulation. These findings provide insights into the mechanisms of cellular activation-mediated HIV-1 production in CD4+ T cells.
Keywords: antiviral immunity; restriction factor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
MCPIP1/regnase-I inhibits simian immunodeficiency virus and is not counteracted by Vpx.J Gen Virol. 2016 Jul;97(7):1693-1698. doi: 10.1099/jgv.0.000482. Epub 2016 Apr 13. J Gen Virol. 2016. PMID: 27075251 Free PMC article.
-
Increased expression of CDKN1A/p21 in HIV-1 controllers is correlated with upregulation of ZC3H12A/MCPIP1.Retrovirology. 2020 Jul 2;17(1):18. doi: 10.1186/s12977-020-00522-4. Retrovirology. 2020. PMID: 32615986 Free PMC article.
-
RGS2 suppresses breast cancer cell growth via a MCPIP1-dependent pathway.J Cell Biochem. 2015 Feb;116(2):260-7. doi: 10.1002/jcb.24964. J Cell Biochem. 2015. PMID: 25187114
-
Monocyte chemotactic protein-1-induced protein-1 (MCPIP1) is a novel multifunctional modulator of inflammatory reactions.Biochim Biophys Acta. 2012 Oct;1823(10):1905-13. doi: 10.1016/j.bbamcr.2012.06.029. Epub 2012 Jul 4. Biochim Biophys Acta. 2012. PMID: 22771441 Review.
-
MCP-1-induced protein-1, an immune regulator.Protein Cell. 2012 Dec;3(12):903-10. doi: 10.1007/s13238-012-2075-9. Epub 2012 Nov 7. Protein Cell. 2012. PMID: 23132255 Free PMC article. Review.
Cited by
-
Monocyte chemoattractant protein-induced protein 1 directly degrades viral miRNAs with a specific motif and inhibits KSHV infection.Nucleic Acids Res. 2021 May 7;49(8):4456-4471. doi: 10.1093/nar/gkab215. Nucleic Acids Res. 2021. PMID: 33823555 Free PMC article.
-
MCPIP1 Enhances TNF-α-Mediated Apoptosis through Downregulation of the NF-κB/cFLIP Axis.Biology (Basel). 2021 Jul 12;10(7):655. doi: 10.3390/biology10070655. Biology (Basel). 2021. PMID: 34356509 Free PMC article.
-
The Functional Deubiquitinating Enzymes in Control of Innate Antiviral Immunity.Adv Sci (Weinh). 2020 Dec 15;8(2):2002484. doi: 10.1002/advs.202002484. eCollection 2021 Jan. Adv Sci (Weinh). 2020. PMID: 33511009 Free PMC article. Review.
-
Zinc finger protein ZFP36L1 inhibits influenza A virus through translational repression by targeting HA, M and NS RNA transcripts.Nucleic Acids Res. 2020 Jul 27;48(13):7371-7384. doi: 10.1093/nar/gkaa458. Nucleic Acids Res. 2020. PMID: 32556261 Free PMC article.
-
Selective degradation of plasmid-derived mRNAs by MCPIP1 RNase.Biochem J. 2019 Oct 15;476(19):2927-2938. doi: 10.1042/BCJ20190646. Biochem J. 2019. PMID: 31530713 Free PMC article.
References
-
- Sheehy AM, Gaddis NC, Choi JD, Malim MH. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature. 2002;418(6898):646–650. - PubMed
-
- Harris RS, et al. DNA deamination mediates innate immunity to retroviral infection. Cell. 2003;113(6):803–809. - PubMed
-
- Mangeat B, et al. Broad antiretroviral defence by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature. 2003;424(6944):99–103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

