Ear acupressure for smoking cessation: a randomised controlled trial
- PMID: 24191168
- PMCID: PMC3804399
- DOI: 10.1155/2013/637073
Ear acupressure for smoking cessation: a randomised controlled trial
Abstract
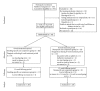
This study investigated the efficacy and safety of ear acupressure (EAP) as a stand-alone intervention for smoking cessation and the feasibility of this study design. Adult smokers were randomised to receive EAP specific for smoking cessation (SSEAP) or a nonspecific EAP (NSEAP) intervention which is not typically used for smoking cessation. Participants received 8 weekly treatments and were requested to press the five pellets taped to one ear at least three times daily. Participants were followed up for three months. Primary outcome measures were a 7-day point-prevalence cessation rate confirmed by exhaled carbon monoxide and relief of nicotine withdrawal symptoms (NWS). Intention-to-treat analysis was applied. Forty-three adult smokers were randomly assigned to SSEAP (n = 20) or NSEAP (n = 23) groups. The dropout rate was high with 19 participants completing the treatments and 12 remaining at followup. One participant from the SSEAP group had confirmed cessation at week 8 and end of followup (5%), but there was no difference between groups for confirmed cessation or NWS. Adverse events were few and minor.
Figures
References
-
- WHO. Why Tobacco is a Public Health Priority. 2013, http://www.who.int/tobacco/health_priority/en/
-
- WHO. Tobacco Fact Sheet. 2012, http://www.who.int/mediacentre/factsheets/fs339/en/index.html.
-
- Shiffman S, Brockwell SE, Pillitteri JL, Gitchell JG. Use of smoking-cessation treatments in the United States. American Journal of Preventive Medicine. 2008;34(2):102–111. - PubMed
-
- Morrell HER, Cohen LM, al’Absi M. Physiological and psychological symptoms and predictors in early nicotine withdrawal. Pharmacology Biochemistry and Behavior. 2008;89(3):272–278. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources