Mycobacterium avium biofilm attenuates mononuclear phagocyte function by triggering hyperstimulation and apoptosis during early infection
- PMID: 24191301
- PMCID: PMC3911830
- DOI: 10.1128/IAI.00820-13
Mycobacterium avium biofilm attenuates mononuclear phagocyte function by triggering hyperstimulation and apoptosis during early infection
Abstract
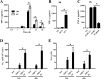
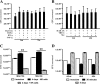
Mycobacterium avium subsp. hominissuis is an opportunistic human pathogen that has been shown to form biofilm in vitro and in vivo. Biofilm formation in vivo appears to be associated with infections in the respiratory tract of the host. The reasoning behind how M. avium subsp. hominissuis biofilm is allowed to establish and persist without being cleared by the innate immune system is currently unknown. To identify the mechanism responsible for this, we developed an in vitro model using THP-1 human mononuclear phagocytes cocultured with established M. avium subsp. hominissuis biofilm and surveyed various aspects of the interaction, including phagocyte stimulation and response, bacterial killing, and apoptosis. M. avium subsp. hominissuis biofilm triggered robust tumor necrosis factor alpha (TNF-α) release from THP-1 cells as well as superoxide and nitric oxide production. Surprisingly, the hyperstimulated phagocytes did not effectively eliminate the cells of the biofilm, even when prestimulated with gamma interferon (IFN-γ) or TNF-α or cocultured with natural killer cells (which have been shown to induce anti-M. avium subsp. hominissuis activity when added to THP-1 cells infected with planktonic M. avium subsp. hominissuis). Time-lapse microscopy and the TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling) assay determined that contact with the M. avium subsp. hominissuis biofilm led to early, widespread onset of apoptosis, which is not seen until much later in planktonic M. avium subsp. hominissuis infection. Blocking TNF-α or TNF-R1 during interaction with the biofilm significantly reduced THP-1 apoptosis but did not lead to elimination of M. avium subsp. hominissuis. Our data collectively indicate that M. avium subsp. hominissuis biofilm induces TNF-α-driven hyperstimulation and apoptosis of surveilling phagocytes, which prevents clearance of the biofilm by cells of the innate immune system and allows the biofilm-associated infection to persist.
Figures


Similar articles
-
Mycobacterium avium subsp. hominissuis effector MAVA5_06970 promotes rapid apoptosis in secondary-infected macrophages during cell-to-cell spread.Virulence. 2018;9(1):1287-1300. doi: 10.1080/21505594.2018.1504559. Virulence. 2018. PMID: 30134761 Free PMC article.
-
The environment of "Mycobacterium avium subsp. hominissuis" microaggregates induces synthesis of small proteins associated with efficient infection of respiratory epithelial cells.Infect Immun. 2015 Feb;83(2):625-36. doi: 10.1128/IAI.02699-14. Epub 2014 Nov 24. Infect Immun. 2015. PMID: 25422262 Free PMC article.
-
Mycobacterium avium subsp. avium and subsp. hominissuis give different cytokine responses after in vitro stimulation of human blood mononuclear cells.PLoS One. 2012;7(4):e34391. doi: 10.1371/journal.pone.0034391. Epub 2012 Apr 10. PLoS One. 2012. PMID: 22506018 Free PMC article.
-
Immunobiology of Mycobacterium avium infection.Eur J Clin Microbiol Infect Dis. 1994 Nov;13(11):1000-6. doi: 10.1007/BF02111501. Eur J Clin Microbiol Infect Dis. 1994. PMID: 7698113 Review.
-
Dose response models and a quantitative microbial risk assessment framework for the Mycobacterium avium complex that account for recent developments in molecular biology, taxonomy, and epidemiology.Water Res. 2017 Feb 1;109:310-326. doi: 10.1016/j.watres.2016.11.053. Epub 2016 Nov 24. Water Res. 2017. PMID: 27915187 Review.
Cited by
-
Immunological and microbial shifts in the aging rhesus macaque lung during nontuberculous mycobacterial infection.mBio. 2024 Jun 12;15(6):e0082924. doi: 10.1128/mbio.00829-24. Epub 2024 May 21. mBio. 2024. PMID: 38771046 Free PMC article.
-
Discovery of Biofilm-Inhibiting Compounds to Enhance Antibiotic Effectiveness Against M. abscessus Infections.Pharmaceuticals (Basel). 2025 Feb 7;18(2):225. doi: 10.3390/ph18020225. Pharmaceuticals (Basel). 2025. PMID: 40006039 Free PMC article.
-
Mycobacterium avium Possesses Extracellular DNA that Contributes to Biofilm Formation, Structural Integrity, and Tolerance to Antibiotics.PLoS One. 2015 May 26;10(5):e0128772. doi: 10.1371/journal.pone.0128772. eCollection 2015. PLoS One. 2015. PMID: 26010725 Free PMC article.
-
Short-Chain Fatty Acids Promote Mycobacterium avium subsp. hominissuis Growth in Nutrient-Limited Environments and Influence Susceptibility to Antibiotics.Pathogens. 2020 Aug 26;9(9):700. doi: 10.3390/pathogens9090700. Pathogens. 2020. PMID: 32859077 Free PMC article.
-
The complexities and challenges of preventing and treating nontuberculous mycobacterial diseases.PLoS Negl Trop Dis. 2019 Feb 14;13(2):e0007083. doi: 10.1371/journal.pntd.0007083. eCollection 2019 Feb. PLoS Negl Trop Dis. 2019. PMID: 30763316 Free PMC article. Review.
References
-
- Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ, Jr, Winthrop K. 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 175:367–416. 10.1164/rccm.200604-571ST - DOI - PubMed
-
- Nishiuchi Y, Maekura R, Kitada S, Tamaru A, Taguri T, Kira Y, Hiraga T, Hirotani A, Yoshimura K, Miki M, Ito M. 2007. The recovery of Mycobacterium avium-intracellulare complex (MAC) from the residential bathrooms of patients with pulmonary MAC. Clin. Infect. Dis. 45:347–351. 10.1086/519383 - DOI - PubMed
-
- Nishiuchi Y, Tamura A, Kitada S, Taguri T, Matsumoto S, Tateishi Y, Yoshimura M, Ozeki Y, Matsumura N, Ogura H, Maekura R. 2009. Mycobacterium avium complex organisms predominantly colonize in the bathtub inlets of patients' bathrooms. Jpn. J. Infect. Dis. 62:182–186 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

