In vivo roles of conjugation with glutathione and O6-alkylguanine DNA-alkyltransferase in the mutagenicity of the bis-electrophiles 1,2-dibromoethane and 1,2,3,4-diepoxybutane in mice
- PMID: 24191644
- PMCID: PMC3889014
- DOI: 10.1021/tx4003534
In vivo roles of conjugation with glutathione and O6-alkylguanine DNA-alkyltransferase in the mutagenicity of the bis-electrophiles 1,2-dibromoethane and 1,2,3,4-diepoxybutane in mice
Abstract
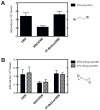
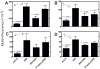
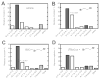
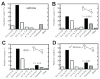
Several studies with bacteria and in vitro mammalian systems have provided evidence of the roles of two thiol-based conjugation systems, glutathione (GSH) transferase and O(6)-alkylguanine DNA-alkyltransferase (AGT), in the bioactivation of the bis-electrophiles 1,2-dibromoethane and 1,2,3,4-diepoxybutane (DEB), the latter an oxidation product of 1,3-butadiene. The in vivo relevance of these conjugation reactions to biological activity in mammals has not been addressed, particularly with DEB. In this work, we used transgenic Big Blue mice, utilizing the cII gene, to examine the effects of manipulation of conjugation pathways on liver mutations arising from dibromoethane and DEB in vivo. Treatment of the mice with butathionine sulfoxime (BSO) prior to dibromoethane lowered hepatic GSH levels, dibromoethane-GSH DNA adduct levels (N(7)-guanyl), and the cII mutation frequency. Administration of O(6)-benzylguanine (O(6)-BzGua), an inhibitor of AGT, did not change the mutation frequency. Depletion of GSH (BSO) and AGT (O(6)-BzGua) lowered the mutation frequency induced by DEB, and BSO lowered the levels of GSH-DEB N(7)-guanyl and N(6)-adenyl DNA adducts. Our results provide evidence that the GSH conjugation pathway is a major in vivo factor in dibromoethane genotoxicity; both GSH conjugation and AGT conjugation are major factors in the genotoxicity of DEB. The latter findings are considered to be relevant to the carcinogenicity of 1,3-butadiene.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Mutation spectra of S-(2-hydroxy-3,4-epoxybutyl)glutathione: comparison with 1,3-butadiene and its metabolites in the Escherichia coli rpoB gene.Chem Res Toxicol. 2012 Jul 16;25(7):1522-30. doi: 10.1021/tx3002109. Epub 2012 Jun 15. Chem Res Toxicol. 2012. PMID: 22670845 Free PMC article.
-
Reactions of glyceraldehyde 3-phosphate dehydrogenase sulfhydryl groups with bis-electrophiles produce DNA-protein cross-links but not mutations.Chem Res Toxicol. 2008 Feb;21(2):453-8. doi: 10.1021/tx7003618. Epub 2007 Dec 29. Chem Res Toxicol. 2008. PMID: 18163542 Free PMC article.
-
Conjugation of butadiene diepoxide with glutathione yields DNA adducts in vitro and in vivo.Chem Res Toxicol. 2012 Mar 19;25(3):706-12. doi: 10.1021/tx200471x. Epub 2012 Jan 9. Chem Res Toxicol. 2012. PMID: 22181695 Free PMC article.
-
Principles of covalent binding of reactive metabolites and examples of activation of bis-electrophiles by conjugation.Arch Biochem Biophys. 2005 Jan 15;433(2):369-78. doi: 10.1016/j.abb.2004.07.035. Arch Biochem Biophys. 2005. PMID: 15581593 Review.
-
Activation of dihaloalkanes by thiol-dependent mechanisms.J Biochem Mol Biol. 2003 Jan 31;36(1):20-7. doi: 10.5483/bmbrep.2003.36.1.020. J Biochem Mol Biol. 2003. PMID: 12542971 Review.
Cited by
-
Formation of S-[2-(N6-Deoxyadenosinyl)ethyl]glutathione in DNA and Replication Past the Adduct by Translesion DNA Polymerases.Chem Res Toxicol. 2017 May 15;30(5):1188-1196. doi: 10.1021/acs.chemrestox.7b00022. Epub 2017 Apr 14. Chem Res Toxicol. 2017. PMID: 28395138 Free PMC article.
-
Protective Role of Glutathione against Peroxynitrite-Mediated DNA Damage During Acute Inflammation.Chem Res Toxicol. 2020 Oct 19;33(10):2668-2674. doi: 10.1021/acs.chemrestox.0c00299. Epub 2020 Sep 18. Chem Res Toxicol. 2020. PMID: 32894672 Free PMC article.
-
Enzymatic bypass of an N6-deoxyadenosine DNA-ethylene dibromide-peptide cross-link by translesion DNA polymerases.J Biol Chem. 2021 Jan-Jun;296:100444. doi: 10.1016/j.jbc.2021.100444. Epub 2021 Feb 20. J Biol Chem. 2021. PMID: 33617883 Free PMC article.
References
-
- Wong LCK, Winston JM, Hong CB, Plotnick H. Carcinogenicity and toxicity of 1,2-dibromoethane in the rat. Toxicol Appl Pharmacol. 1982;63:155–165. - PubMed
-
- . Ethylene dibromide: worker exposure, use restricted. Chem Eng News. 1983;61:4.
-
- Agency for Toxic Toxic Substances & Disease Registry. [accessed 26 Sept. 2013];Toxic Substances Portal - 1,2-Dibromoethane. http://www.atsdr.cdc.gov/toxprofiles/TP.asp?id=726&tid=131.
-
- [accessed 24 Oct. 2013];Agents Classified by the IARC Monographs. 1–108 http://monographs.iarc.fr/ENG/Classification/
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

