Intrinsic disorder within an AKAP-protein kinase A complex guides local substrate phosphorylation
- PMID: 24192038
- PMCID: PMC3814001
- DOI: 10.7554/eLife.01319
Intrinsic disorder within an AKAP-protein kinase A complex guides local substrate phosphorylation
Abstract
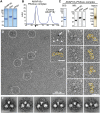
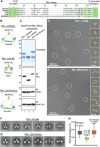
Anchoring proteins sequester kinases with their substrates to locally disseminate intracellular signals and avert indiscriminate transmission of these responses throughout the cell. Mechanistic understanding of this process is hampered by limited structural information on these macromolecular complexes. A-kinase anchoring proteins (AKAPs) spatially constrain phosphorylation by cAMP-dependent protein kinases (PKA). Electron microscopy and three-dimensional reconstructions of type-II PKA-AKAP18γ complexes reveal hetero-pentameric assemblies that adopt a range of flexible tripartite configurations. Intrinsically disordered regions within each PKA regulatory subunit impart the molecular plasticity that affords an ∼16 nanometer radius of motion to the associated catalytic subunits. Manipulating flexibility within the PKA holoenzyme augmented basal and cAMP responsive phosphorylation of AKAP-associated substrates. Cell-based analyses suggest that the catalytic subunit remains within type-II PKA-AKAP18γ complexes upon cAMP elevation. We propose that the dynamic movement of kinase sub-structures, in concert with the static AKAP-regulatory subunit interface, generates a solid-state signaling microenvironment for substrate phosphorylation. DOI: http://dx.doi.org/10.7554/eLife.01319.001.
Keywords: A-kinase anchoring protein (AKAP); cAMP signaling; cAMP-dependent kinase (PKA); electron microscopy; intrinsic disorder; single particle reconstruction.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures







References
-
- Bouvier M, Leeb-Lundberg LM, Benovic JL, Caron MG, Lefkowitz RJ. 1987. Regulation of adrenergic receptor function by phosphorylation. II. Effects of agonist occupancy on phosphorylation of alpha 1- and beta 2- adrenergic receptors by protein kinase C and the cyclic AMP-dependent protein kinase. J Biol Chem 262:3106–13 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

