Intraspinal transplantation and modulation of donor neuron electrophysiological activity
- PMID: 24192152
- PMCID: PMC3893055
- DOI: 10.1016/j.expneurol.2013.10.016
Intraspinal transplantation and modulation of donor neuron electrophysiological activity
Abstract
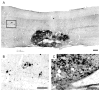
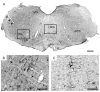
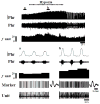
Rat fetal spinal cord (FSC) tissue, naturally enriched with interneuronal progenitors, was introduced into high cervical, hemi-resection (Hx) lesions. Electrophysiological analyses were conducted to determine if such grafts exhibit physiologically-patterned neuronal activity and if stimuli which increase respiratory motor output also alter donor neuron bursting. Three months following transplantation, the bursting activity of FSC neurons and the contralateral phrenic nerve were recorded in anesthetized rats during a normoxic baseline period and brief respiratory challenges. Spontaneous neuronal activity was detected in 80% of the FSC transplants, and autocorrelation of action potential spikes revealed distinct correlogram peaks in 87% of neurons. At baseline, the average discharge frequency of graft neurons was 13.0 ± 1.7 Hz, and discharge frequency increased during a hypoxic respiratory challenge (p<0.001). Parallel studies in unanesthetized rats showed that FSC tissue recipients had larger inspiratory tidal volumes during brief hypoxic exposures (p<0.05 vs. C2Hx rats). Anatomical connectivity was explored in additional graft recipients by injecting a transsynaptic retrograde viral tracer (pseudorabies virus, PRV) directly into matured transplants. Neuronal labeling occurred throughout graft tissues and also in the host spinal cord and brainstem nuclei, including those associated with respiratory control. These results underscore the neuroplastic potential of host-graft interactions and training approaches to enhance functional integration within targeted spinal circuitry.
Keywords: Cervical spinal cord injury; Fetal spinal cord; Hypoxia; Respiratory recovery; Spinal cord repair; Transplantation.
© 2013.
Figures



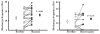
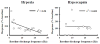
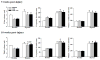
Similar articles
-
Respiratory outcomes after mid-cervical transplantation of embryonic medullary cells in rats with cervical spinal cord injury.Exp Neurol. 2016 Apr;278:22-6. doi: 10.1016/j.expneurol.2016.01.017. Epub 2016 Jan 22. Exp Neurol. 2016. PMID: 26808660 Free PMC article.
-
Respiratory motor outputs following unilateral midcervical spinal cord injury in the adult rat.J Appl Physiol (1985). 2014 Feb 15;116(4):395-405. doi: 10.1152/japplphysiol.01001.2013. Epub 2013 Nov 27. J Appl Physiol (1985). 2014. PMID: 24285148
-
Intraspinal transplantation of subventricular zone-derived neural progenitor cells improves phrenic motor output after high cervical spinal cord injury.Exp Neurol. 2017 Jan;287(Pt 2):205-215. doi: 10.1016/j.expneurol.2016.06.007. Epub 2016 Jun 11. Exp Neurol. 2017. PMID: 27302679 Free PMC article.
-
Fetal cell grafts into resection and contusion/compression injuries of the rat and cat spinal cord.Exp Neurol. 1992 Jan;115(1):177-88. doi: 10.1016/0014-4886(92)90245-l. Exp Neurol. 1992. PMID: 1370221 Review.
-
Transplant therapy: recovery of function after spinal cord injury.J Neurotrauma. 1997 Aug;14(8):479-506. doi: 10.1089/neu.1997.14.479. J Neurotrauma. 1997. PMID: 9300561 Review.
Cited by
-
Intermittent hypoxia and neurorehabilitation.J Appl Physiol (1985). 2015 Dec 15;119(12):1455-65. doi: 10.1152/japplphysiol.00235.2015. Epub 2015 May 21. J Appl Physiol (1985). 2015. PMID: 25997947 Free PMC article. Review.
-
Transplanting Cells for Spinal Cord Repair: Who, What, When, Where and Why?Cell Transplant. 2019 Apr;28(4):388-399. doi: 10.1177/0963689718824097. Epub 2019 Jan 18. Cell Transplant. 2019. PMID: 30654638 Free PMC article.
-
Neural Stem Cells: Promoting Axonal Regeneration and Spinal Cord Connectivity.Cells. 2021 Nov 25;10(12):3296. doi: 10.3390/cells10123296. Cells. 2021. PMID: 34943804 Free PMC article. Review.
-
Transplantation of Neural Progenitors and V2a Interneurons after Spinal Cord Injury.J Neurotrauma. 2018 Dec 15;35(24):2883-2903. doi: 10.1089/neu.2017.5439. Epub 2018 Aug 10. J Neurotrauma. 2018. PMID: 29873284 Free PMC article.
-
Transplanting neural progenitor cells to restore connectivity after spinal cord injury.Nat Rev Neurosci. 2020 Jul;21(7):366-383. doi: 10.1038/s41583-020-0314-2. Epub 2020 Jun 9. Nat Rev Neurosci. 2020. PMID: 32518349 Free PMC article. Review.
References
-
- Auerbach JM, Eiden MV, McKay RD. Transplanted CNS stem cells form functional synapses in vivo. Eur J Neurosci. 2000;12:1696–1704. - PubMed
-
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci. 2004;7:48–55. - PubMed
-
- Bar-Gad I, Ritov Y, Bergman H. The neuronal refractory period causes a short-term peak in the autocorrelation function. Journal of neuroscience methods. 2001;104:155–163. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

