A family portrait: structural comparison of the Whirly proteins from Arabidopsis thaliana and Solanum tuberosum
- PMID: 24192350
- PMCID: PMC3818034
- DOI: 10.1107/S1744309113028698
A family portrait: structural comparison of the Whirly proteins from Arabidopsis thaliana and Solanum tuberosum
Abstract
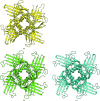
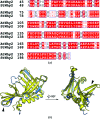
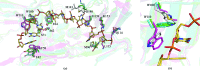
DNA double-strand breaks are highly detrimental genomic lesions that routinely arise in genomes. To protect the integrity of their genetic information, all organisms have evolved specialized DNA-repair mechanisms. Whirly proteins modulate DNA repair in plant chloroplasts and mitochondria by binding single-stranded DNA in a non-sequence-specific manner. Although most of the results showing the involvement of the Whirly proteins in DNA repair have been obtained in Arabidopsis thaliana, only the crystal structures of the potato Whirly proteins WHY1 and WHY2 have been reported to date. The present report of the crystal structures of the three Whirly proteins from A. thaliana (WHY1, WHY2 and WHY3) reveals that these structurally similar proteins assemble into tetramers. Furthermore, structural alignment with a potato WHY2-DNA complex reveals that the residues in these proteins are properly oriented to bind single-stranded DNA in a non-sequence-specific manner.
Keywords: Arabidopsis; DNA repair; WHY1; WHY2; WHY3; Whirly proteins; ssDNA-binding proteins.
Figures

References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

