Selective neuronal loss in ischemic stroke and cerebrovascular disease
- PMID: 24192635
- PMCID: PMC3887360
- DOI: 10.1038/jcbfm.2013.188
Selective neuronal loss in ischemic stroke and cerebrovascular disease
Abstract
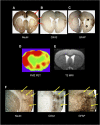
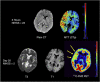
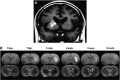
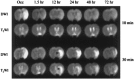
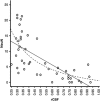
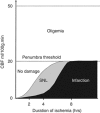
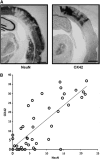
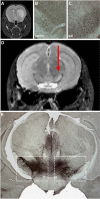
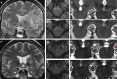
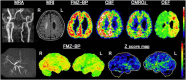
As a sequel of brain ischemia, selective neuronal loss (SNL)-as opposed to pannecrosis (i.e. infarction)-is attracting growing interest, particularly because it is now detectable in vivo. In acute stroke, SNL may affect the salvaged penumbra and hamper functional recovery following reperfusion. Rodent occlusion models can generate SNL predominantly in the striatum or cortex, showing that it can affect behavior for weeks despite normal magnetic resonance imaging. In humans, SNL in the salvaged penumbra has been documented in vivo mainly using positron emission tomography and (11)C-flumazenil, a neuronal tracer validated against immunohistochemistry in rodent stroke models. Cortical SNL has also been documented using this approach in chronic carotid disease in association with misery perfusion and behavioral deficits, suggesting that it can result from chronic or unstable hemodynamic compromise. Given these consequences, SNL may constitute a novel therapeutic target. Selective neuronal loss may also develop at sites remote from infarcts, representing secondary 'exofocal' phenomena akin to degeneration, potentially related to poststroke behavioral or mood impairments again amenable to therapy. Further work should aim to better characterize the time course, behavioral consequences-including the impact on neurological recovery and contribution to vascular cognitive impairment-association with possible causal processes such as microglial activation, and preventability of SNL.
Figures
References
-
- Graham DI, Peter PL.Greenfield's Neuropathology7th ednArnold publisher: London; 20021251–258.
-
- Garcia JH, Lassen NA, Weiller C, Sperling B, Nakagawara J. Ischemic stroke and incomplete infarction. Stroke. 1996;27:761–765. - PubMed
-
- DeGirolami U, Crowell RM, Marcoux FW. Selective necrosis and total necrosis in focal cerebral ischemia. Neuropathologic observations on experimental middle cerebral artery occlusion in the macaque monkey. J Neuropathol Exp Neurol. 1984;43:57–71. - PubMed
-
- Auer RN, Siesjo BK. Biological differences between ischemia, hypoglycemia, and epilepsy. Ann Neurol. 1988;24:699–707. - PubMed
-
- Spielmeyer W. Zur pathogenese örtlich elektiver gehirnveränderungen. Z Ges Neurol Psych. 1925;99:756–776.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

