Developmental origins of nonalcoholic fatty liver disease
- PMID: 24192698
- PMCID: PMC4081536
- DOI: 10.1038/pr.2013.193
Developmental origins of nonalcoholic fatty liver disease
Abstract
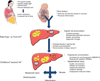
Obese pregnant women may transmit their metabolic phenotype to offspring, leading to a cycle of obesity and diabetes over generations. Early childhood obesity predicts nonalcoholic fatty liver disease (NAFLD), the most common chronic human liver disease. The fetus may be vulnerable to steatosis because immature fetal adipose depots are not available to buffer the excess transplacental lipid delivery in maternal obesity. In animal models, in utero high-fat diet exposure results in an increase in the accumulation of liver triglycerides in offspring and increased hepatic oxidative stress and apoptosis, perhaps priming the liver for later development of NAFLD. Innate immune dysfunction and necroinflammatory changes have been observed in postnatal offspring liver of animals born to high-fat-fed dams. Postweaning, livers of offspring exposed to maternal high-fat feeding in utero share pathophysiologic features with human NAFLD, including increased de novo lipogenesis and decreased free fatty acid oxidation. Human studies using magnetic resonance imaging have shown that maternal BMI predicts infant intrahepatocellular lipid storage, as seen in animal models. The generational transfer of NAFLD may occur via epigenetic changes in offspring liver. Transmission of microbiota from mother to infant may impact energy retention and immune function that contribute to a predisposition to NAFLD.
Figures

References
-
- Agopian VG, Kaldas FM, Hong JC, et al. Liver transplantation for nonalcoholic steatohepatitis: the new epidemic. Ann Surg. 2012;256:624–633. - PubMed
-
- Alisi A, Manco M, Vania A, Nobili V. Pediatric nonalcoholic fatty liver disease in 2009. J Pediatr. 2009;155:469–474. - PubMed
-
- Schwimmer JB, McGreal N, Deutsch R, Finegold MJ, Lavine JE. Influence of gender, race, and ethnicity on suspected fatty liver in obese adolescents. Pediatrics. 2005;115:e561–e565. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

