Oncogenic function of SCCRO5/DCUN1D5 requires its Neddylation E3 activity and nuclear localization
- PMID: 24192928
- PMCID: PMC4191716
- DOI: 10.1158/1078-0432.CCR-13-1252
Oncogenic function of SCCRO5/DCUN1D5 requires its Neddylation E3 activity and nuclear localization
Abstract
Purpose: To determine mechanisms by which SCCRO5 (aka DCUN1D5) promotes oncogenesis.
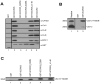
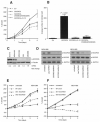
Experimental design: SCCRO5 mRNA and protein expression were assessed in 203 randomly selected primary cancer tissue samples, matched histologically normal tissues, and cell lines by use of real-time PCR and Western blot analysis. SCCRO5 overexpression was correlated with survival. The effect of SCCRO5 knockdown on viability was assessed in selected cancer cell lines. Structure-function studies were performed to determine the SCCRO5 residues required for binding to the neddylation components, for neddylation-promoting activity, and for transformation.
Results: In oral and lung squamous cell carcinomas, SCCRO5 mRNA levels corresponded with protein levels and overexpression correlated with decreased disease-specific survival. Knockdown of SCCRO5 by RNAi resulted in a selective decrease in the viability of cancer cells with high endogenous levels, suggesting the presence of oncogene addiction. SCCRO5 promoted cullin neddylation while maintaining conserved reaction processivity paradigms involved in ubiquitin and ubiquitin-like protein conjugation, establishing it as a component of the neddylation E3. Neddylation activities in vitro required the potentiating of neddylation (PONY) domain but not the nuclear localization sequence (NLS) domain. In contrast, both the NLS domain and the PONY domain were required for transformation of NIH-3T3 cells.
Conclusions: Our data suggest that SCCRO5 has oncogenic potential that requires its function as a component of the neddylation E3. Neddylation activity and nuclear localization of SCCRO5 are important for its in vivo function.
©2013 AACR.
Figures
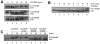

References
-
- Hsu JM, Lee YC, Yu CT, Huang CY. Fbx7 functions in the SCF complex regulating Cdk1-cyclin B-phosphorylated hepatoma up-regulated protein (HURP) proteolysis by a proline-rich region. J Biol Chem. 2004;279:32592–602. - PubMed
-
- Lehman NL, van de Rijn M, Jackson PK. Screening of tissue microarrays for ubiquitin proteasome system components in tumors. Methods Enzymol. 2005;399:334–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

