EGFR-Targeted Adenovirus Dendrimer Coating for Improved Systemic Delivery of the Theranostic NIS Gene
- PMID: 24193032
- PMCID: PMC3889187
- DOI: 10.1038/mtna.2013.58
EGFR-Targeted Adenovirus Dendrimer Coating for Improved Systemic Delivery of the Theranostic NIS Gene
Abstract
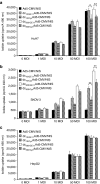
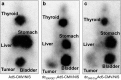
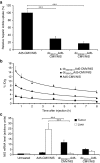
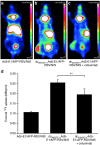
We recently demonstrated tumor-selective iodide uptake and therapeutic efficacy of combined radiovirotherapy after systemic delivery of the theranostic sodium iodide symporter (NIS) gene using a dendrimer-coated adenovirus. To further improve shielding and targeting we physically coated replication-selective adenoviruses carrying the hNIS gene with a conjugate consisting of cationic poly(amidoamine) (PAMAM) dendrimer linked to the peptidic, epidermal growth factor receptor (EGFR)-specific ligand GE11. In vitro experiments demonstrated coxsackie-adenovirus receptor-independent but EGFR-specific transduction efficiency. Systemic injection of the uncoated adenovirus in a liver cancer xenograft mouse model led to high levels of NIS expression in the liver due to hepatic sequestration, which were significantly reduced after coating as demonstrated by (123)I-scintigraphy. Reduction of adenovirus liver pooling resulted in decreased hepatotoxicity and increased transduction efficiency in peripheral xenograft tumors. (124)I-PET-imaging confirmed EGFR-specificity by significantly lower tumoral radioiodine accumulation after pretreatment with the EGFR-specific antibody cetuximab. A significantly enhanced oncolytic effect was observed following systemic application of dendrimer-coated adenovirus that was further increased by additional treatment with a therapeutic dose of (131)I. These results demonstrate restricted virus tropism and tumor-selective retargeting after systemic application of coated, EGFR-targeted adenoviruses therefore representing a promising strategy for improved systemic adenoviral NIS gene therapy.Molecular Therapy-Nucleic Acids (2013) 2, e131; doi:10.1038/mtna.2013.58; published online 5 November 2013.
Figures

Similar articles
-
Systemic image-guided liver cancer radiovirotherapy using dendrimer-coated adenovirus encoding the sodium iodide symporter as theranostic gene.J Nucl Med. 2013 Aug;54(8):1450-7. doi: 10.2967/jnumed.112.115493. Epub 2013 Jul 10. J Nucl Med. 2013. PMID: 23843567
-
Imaging and targeted therapy of pancreatic ductal adenocarcinoma using the theranostic sodium iodide symporter (NIS) gene.Oncotarget. 2017 May 16;8(20):33393-33404. doi: 10.18632/oncotarget.16499. Oncotarget. 2017. PMID: 28380420 Free PMC article.
-
Epidermal growth factor receptor-targeted (131)I-therapy of liver cancer following systemic delivery of the sodium iodide symporter gene.Mol Ther. 2011 Apr;19(4):676-85. doi: 10.1038/mt.2010.296. Epub 2011 Jan 18. Mol Ther. 2011. PMID: 21245850 Free PMC article.
-
The sodium iodide symporter (NIS): novel applications for radionuclide imaging and treatment.Endocr Relat Cancer. 2021 Sep 3;28(10):T193-T213. doi: 10.1530/ERC-21-0177. Endocr Relat Cancer. 2021. PMID: 34259647 Review.
-
A transporter gene (sodium iodide symporter) for dual purposes in gene therapy: imaging and therapy.Curr Gene Ther. 2002 Dec;2(4):393-402. doi: 10.2174/1566523023347599. Curr Gene Ther. 2002. PMID: 12477251 Review.
Cited by
-
Polymeric oncolytic adenovirus for cancer gene therapy.J Control Release. 2015 Dec 10;219:181-191. doi: 10.1016/j.jconrel.2015.10.009. Epub 2015 Oct 23. J Control Release. 2015. PMID: 26453806 Free PMC article. Review.
-
Radiocobalt-Labeling of a Polypyridylamine Chelate Conjugated to GE11 for EGFR-Targeted Theranostics.Molecules. 2025 Jan 7;30(2):212. doi: 10.3390/molecules30020212. Molecules. 2025. PMID: 39860082 Free PMC article.
-
Systemic administration of human mesenchymal stromal cells infected with polymer-coated oncolytic adenovirus induces efficient pancreatic tumor homing and infiltration.J Control Release. 2019 Jul 10;305:75-88. doi: 10.1016/j.jconrel.2019.04.040. Epub 2019 May 6. J Control Release. 2019. PMID: 31071373 Free PMC article.
-
Effective control of tumor growth through spatial and temporal control of theranostic sodium iodide symporter (NIS) gene expression using a heat-inducible gene promoter in engineered mesenchymal stem cells.Theranostics. 2020 Mar 15;10(10):4490-4506. doi: 10.7150/thno.41489. eCollection 2020. Theranostics. 2020. PMID: 32292510 Free PMC article.
-
Nano-engineered delivery systems for cancer imaging and therapy: Recent advances, future direction and patent evaluation.Drug Discov Today. 2019 Feb;24(2):462-491. doi: 10.1016/j.drudis.2018.08.009. Epub 2018 Aug 16. Drug Discov Today. 2019. PMID: 30121330 Free PMC article. Review.
References
-
- Grünwald GK, Vetter A, Klutz K, Willhauck MJ, Schwenk N, Senekowitsch-Schmidtke R, et al. Systemic image-guided liver cancer radiovirotherapy using dendrimer-coated adenovirus encoding the sodium iodide symporter as theranostic gene. J Nucl Med. 2013;54:1450–1457. - PubMed
-
- Vetter A, Virdi KS, Espenlaub S, Rödl W, Wagner E, Holm PS, et al. Adenoviral vectors coated with PAMAM dendrimer conjugates allow CAR independent virus uptake and targeting to the EGF receptor. Mol Pharm. 2013;10:606–618. - PubMed
-
- Choi JW, Lee JS, Kim SW, Yun CO. Evolution of oncolytic adenovirus for cancer treatment. Adv Drug Deliv Rev. 2012;64:720–729. - PubMed
-
- Kim PH, Sohn JH, Choi JW, Jung Y, Kim SW, Haam S, et al. Active targeting and safety profile of PEG-modified adenovirus conjugated with herceptin. Biomaterials. 2011;32:2314–2326. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

