Insights into distinct modulation of α7 and α7β2 nicotinic acetylcholine receptors by the volatile anesthetic isoflurane
- PMID: 24194515
- PMCID: PMC3861630
- DOI: 10.1074/jbc.M113.508333
Insights into distinct modulation of α7 and α7β2 nicotinic acetylcholine receptors by the volatile anesthetic isoflurane
Abstract
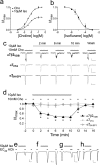
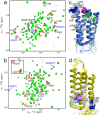
Nicotinic acetylcholine receptors (nAChRs) are targets of general anesthetics, but functional sensitivity to anesthetic inhibition varies dramatically among different subtypes of nAChRs. Potential causes underlying different functional responses to anesthetics remain elusive. Here we show that in contrast to the α7 nAChR, the α7β2 nAChR is highly susceptible to inhibition by the volatile anesthetic isoflurane in electrophysiology measurements. Isoflurane-binding sites in β2 and α7 were found at the extracellular and intracellular end of their respective transmembrane domains using NMR. Functional relevance of the identified β2 site was validated via point mutations and subsequent functional measurements. Consistent with their functional responses to isoflurane, β2 but not α7 showed pronounced dynamics changes, particularly for the channel gate residue Leu-249(9'). These results suggest that anesthetic binding alone is not sufficient to generate functional impact; only those sites that can modulate channel dynamics upon anesthetic binding will produce functional effects.
Keywords: Anesthetics; Cys-loop Receptors; Electrophysiology; Isoflurane; NMR; Nicotinic Acetylcholine Receptors; Protein Dynamics; α7; α7β2.
Figures



References
-
- Palma E., Maggi L., Barabino B., Eusebi F., Ballivet M. (1999) Nicotinic acetylcholine receptors assembled from the α7 and β3 subunits. J. Biol. Chem. 274, 18335–18340 - PubMed
-
- Liu Q., Huang Y., Xue F., Simard A., DeChon J., Li G., Zhang J., Lucero L., Wang M., Sierks M., Hu G., Chang Y., Lukas R. J., Wu J. (2009) A novel nicotinic acetylcholine receptor subtype in basal forebrain cholinergic neurons with high sensitivity to amyloid peptides. J. Neurosci. 29, 918–929 - PMC - PubMed
-
- Picciotto M. R., Zoli M., Léna C., Bessis A., Lallemand Y., Le Novère N., Vincent P., Pich E. M., Brûlet P., Changeux J. P. (1995) Abnormal avoidance learning in mice lacking functional high-affinity nicotine receptor in the brain. Nature 374, 65–67 - PubMed
-
- Marubio L. M., del Mar Arroyo-Jimenez M., Cordero-Erausquin M., Léna C., Le Novère N., de Kerchove d'Exaerde A., Huchet M., Damaj M. I., Changeux J. P. (1999) Reduced antinociception in mice lacking neuronal nicotinic receptor subunits. Nature 398, 805–810 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

