Frequent replenishment sustains the beneficial microbiome of Drosophila melanogaster
- PMID: 24194543
- PMCID: PMC3892787
- DOI: 10.1128/mBio.00860-13
Frequent replenishment sustains the beneficial microbiome of Drosophila melanogaster
Abstract
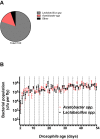
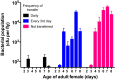
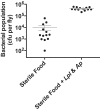
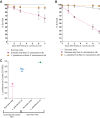
We report that establishment and maintenance of the Drosophila melanogaster microbiome depend on ingestion of bacteria. Frequent transfer of flies to sterile food prevented establishment of the microbiome in newly emerged flies and reduced the predominant members, Acetobacter and Lactobacillus spp., by 10- to 1,000-fold in older flies. Flies with a normal microbiome were less susceptible than germfree flies to infection by Serratia marcescens and Pseudomonas aeruginosa. Augmentation of the normal microbiome with higher populations of Lactobacillus plantarum, a Drosophila commensal and probiotic used in humans, further protected the fly from infection. Replenishment represents an unexplored strategy by which animals can sustain a gut microbial community. Moreover, the population behavior and health benefits of L. plantarum resemble features of certain probiotic bacteria administered to humans. As such, L. plantarum in the fly gut may serve as a simple model for dissecting the population dynamics and mode of action of probiotics in animal hosts.
Importance: Previous studies have defined the composition of the Drosophila melanogaster microbiome in laboratory and wild-caught flies. Our study advances current knowledge in this field by demonstrating that Drosophila must consume bacteria to establish and maintain its microbiome. This finding suggests that the dominant Drosophila symbionts remain associated with their host because of repeated reintroduction rather than internal growth. Furthermore, our study shows that one member of the microbiome, Lactobacillus plantarum, protects the fly from intestinal pathogens. These results suggest that, although not always present, the microbiota can promote salubrious effects for the host. In sum, our work provides a previously unexplored mechanism of microbiome maintenance and an in vivo model system for investigating the mechanisms of action of probiotic bacteria.
Figures

References
-
- Venema K. 2010. Role of gut microbiome in the control of energy and carbohydrate metabolism. Curr. Opin. Clin. Nutr. Metab. Care 13:432–438 - PubMed
-
- Hooper LV, Midtvedt T, Gordon JI. 2002. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu. Rev. Nutr. 22:283–307 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
