Differential increases in the expression of intermediate filament proteins and concomitant morphological changes of transdifferentiating rat hepatic stellate cells observed in vitro
- PMID: 24194627
- PMCID: PMC3813821
- DOI: 10.1267/ahc.13007
Differential increases in the expression of intermediate filament proteins and concomitant morphological changes of transdifferentiating rat hepatic stellate cells observed in vitro
Abstract
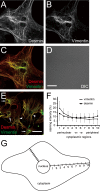
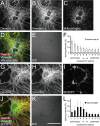
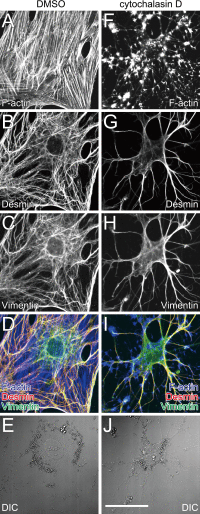
The primary function of hepatic stellate cells (HSCs) is the storage of vitamin A. However, they are also responsible for liver fibrosis and are therapeutic targets for treatment of liver cirrhosis. Among the many molecular markers that define quiescent or activated states of HSCs, the characteristics of type III intermediate filaments are of particular interest. Whereas vimentin and desmin are upregulated in activated HSCs, glial fibrillary acidic protein is downregulated in activated HSCs. The functional differences between vimentin and desmin are poorly understood. By time-course quantifications of several molecular markers for HSC activation, we observed that the expression of vimentin preceded that of desmin during the transdifferentiation of HSCs. The immunoreactivity of vimentin in transdifferentiated HSCs was more intense in perinuclear regions compared to that of desmin. We propose that the delayed expression of desmin following the expression of vimentin and the peripheral localization of desmin compared to vimentin are both related to the more extended phenotype of transdifferentiating HSCs observed in vitro.
Keywords: desmin; hepatic stellate cell; intermediate filament; myofibroblast; vimentin.
Figures


Similar articles
-
Formation of normal desmin intermediate filaments in mouse hepatic stellate cells requires vimentin.Hepatology. 2001 Jan;33(1):177-88. doi: 10.1053/jhep.2001.21045. Hepatology. 2001. PMID: 11124834
-
Class VI intermediate filament protein nestin is induced during activation of rat hepatic stellate cells.Hepatology. 1999 Feb;29(2):520-7. doi: 10.1002/hep.510290232. Hepatology. 1999. PMID: 9918930
-
Intermediate filaments modulation in an in vitro model of the hepatic stellate cell activation or conversion into the lipocyte phenotype.Biochem Cell Biol. 2001;79(4):409-17. Biochem Cell Biol. 2001. PMID: 11527210
-
Wnt signaling in liver fibrosis: progress, challenges and potential directions.Biochimie. 2013 Dec;95(12):2326-35. doi: 10.1016/j.biochi.2013.09.003. Epub 2013 Sep 13. Biochimie. 2013. PMID: 24036368 Review.
-
Apoptotic and survival signals in hepatic stellate cells.Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2007 Oct;32(5):726-34. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2007. PMID: 18007061 Review.
Cited by
-
Establishment and characterization of rat portal myofibroblast cell lines.PLoS One. 2015 Mar 30;10(3):e0121161. doi: 10.1371/journal.pone.0121161. eCollection 2015. PLoS One. 2015. PMID: 25822334 Free PMC article.
-
The HIV matrix protein p17 promotes the activation of human hepatic stellate cells through interactions with CXCR2 and Syndecan-2.PLoS One. 2014 Apr 15;9(4):e94798. doi: 10.1371/journal.pone.0094798. eCollection 2014. PLoS One. 2014. PMID: 24736615 Free PMC article.
References
-
- Ballardini G., Fallani M., Biagini G., Bianchi F. B., Pisi E. Desmin and actin in the identification of Ito cells and in monitoring their evolution to myofibroblasts in experimental liver fibrosis. Virchows Arch. B. Cell Pathol. Incl. Mol. Pathol. 1988;56:45–49. - PubMed
-
- Ballardini G., Groff P., Badiali de Giorgi L., Schuppan D., Bianchi F. B. Ito cell heterogeneity: desmin-negative Ito cells in normal rat liver. Hepatology. 1994;19:440–446. - PubMed
-
- de Leeuw A. M., McCarthy S. P., Geerts A., Knook D. L. Purified rat liver fat-storing cells in culture divide and contain collagen. Hepatology. 1984;4:392–403. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

