Matrix metalloproteinase-2 degrades fibrillin-1 and fibrillin-2 of oxytalan fibers in the human eye and periodontal ligaments in vitro
- PMID: 24194629
- PMCID: PMC3813822
- DOI: 10.1267/ahc.13024
Matrix metalloproteinase-2 degrades fibrillin-1 and fibrillin-2 of oxytalan fibers in the human eye and periodontal ligaments in vitro
Abstract
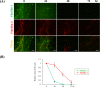
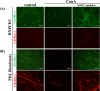
Oxytalan fibers are distributed in the eye and periodontal ligaments (PDL). The ciliary zonule, known as Zinn's zonule, in the eye is composed of oxytalan fibers, which are bundles of microfibrils consisting mainly of fibrillin-1 and fibrillin-2. As turnover of oxytalan fibers is slow during life, their degradation mechanism remains unclarified. This study was performed to examine degradation pattern of fibrillin-1 and fibrillin-2 by experimental MMP activation. We cultured human non-pigmented ciliary epithelial cells (HNPCEC) and PDL fibroblasts for 7 days, then treated them with concanavalin A to activate matrix metalloproteinase (MMP)-2, and examined the degradation of fibrillin-1 and fibrillin-2 for 72 hr using immunofluorescence. At 7 days of HNPCEC culture, fibrillin-1-positive fibers were observed, some of which merged with fibrillin-2. After MMP-2 activation, fibrillin-1-positive fibers became thin and disappeared by 72 hr, while fibrillin-2-positive fibers disappeared almost completely within 24 hr. At 7 days of PDL fibroblast culture, fibrillin-1-positive fibers were mostly merged with fibrillin-2. After MMP-2 activation, fibrillin-1-positive fibers became thin by 24 hr and had almost disappeared by 48 hr, while fibrillin-2-positive fibers decreased constantly after 24 hr. A MMP-2 inhibitor completely suppressed these degradations. These results suggest that the patterns of fibrillin-1 and fibrillin-2 degradation differ between the eye and the PDL, possibly reflecting the sensitivity of fibrillin-1 and fibrillin-2 of each type of oxytalan fiber against MMP-2.
Keywords: ciliary zonule; fibrillin; microfibrils; oxytalan fibers.
Figures


Similar articles
-
Matrix Metalloproteinase-2 Activated by Ultraviolet-B Degrades Human Ciliary Zonules In Vitro.Acta Histochem Cytochem. 2021 Feb 25;54(1):1-9. doi: 10.1267/ahc.20-00021. Epub 2021 Feb 9. Acta Histochem Cytochem. 2021. PMID: 33731965 Free PMC article.
-
Microfibril-associated glycoprotein-1 controls human ciliary zonule development in vitro.Acta Histochem Cytochem. 2014 Feb 27;47(1):11-7. doi: 10.1267/ahc.13038. Epub 2014 Feb 18. Acta Histochem Cytochem. 2014. PMID: 24761045 Free PMC article.
-
Fibrillin-1 and fibrillin-2 are essential for formation of thick oxytalan fibers in human nonpigmented ciliary epithelial cells in vitro.Connect Tissue Res. 2012;53(1):14-20. doi: 10.3109/03008207.2011.602767. Epub 2011 Aug 18. Connect Tissue Res. 2012. PMID: 21851253
-
Zinn's zonule.Prog Retin Eye Res. 2021 May;82:100902. doi: 10.1016/j.preteyeres.2020.100902. Epub 2020 Sep 25. Prog Retin Eye Res. 2021. PMID: 32980533 Free PMC article. Review.
-
The oxytalan fibre network in the periodontium and its possible mechanical function.Arch Oral Biol. 2012 Aug;57(8):1003-11. doi: 10.1016/j.archoralbio.2012.06.003. Epub 2012 Jul 9. Arch Oral Biol. 2012. PMID: 22784380 Review.
Cited by
-
Matrix Metalloproteinase-2 Activated by Ultraviolet-B Degrades Human Ciliary Zonules In Vitro.Acta Histochem Cytochem. 2021 Feb 25;54(1):1-9. doi: 10.1267/ahc.20-00021. Epub 2021 Feb 9. Acta Histochem Cytochem. 2021. PMID: 33731965 Free PMC article.
-
Expression of Lymphatic Markers in the Berger's Space and Bursa Premacularis.Int J Mol Sci. 2021 Feb 19;22(4):2086. doi: 10.3390/ijms22042086. Int J Mol Sci. 2021. PMID: 33669860 Free PMC article.
-
The Effect of Ultraviolet B on Fibrillin-1 and Fibrillin-2 in Human Non-pigmented Ciliary Epithelial Cells In Vitro.Acta Histochem Cytochem. 2017 Jun 26;50(3):105-109. doi: 10.1267/ahc.16036. Epub 2017 Jun 14. Acta Histochem Cytochem. 2017. PMID: 28744027 Free PMC article.
-
Distribution and associations of anterior lens zonules lengths in patients with cataract.Graefes Arch Clin Exp Ophthalmol. 2024 Aug;262(8):2515-2523. doi: 10.1007/s00417-024-06379-z. Epub 2024 Mar 1. Graefes Arch Clin Exp Ophthalmol. 2024. PMID: 38427049
-
Microfibril-associated glycoprotein-1 controls human ciliary zonule development in vitro.Acta Histochem Cytochem. 2014 Feb 27;47(1):11-7. doi: 10.1267/ahc.13038. Epub 2014 Feb 18. Acta Histochem Cytochem. 2014. PMID: 24761045 Free PMC article.
References
-
- Böck P., Stockinger L. Light and electron microscopic identification of elastic, elaunin and oxytalan fibers in human tracheal and bronchial mucosa. Anat. Embryol. (Berl) 1984;170:145–153. - PubMed
-
- Cain S. A., Morgan A., Sherratt M. J., Ball S. G., Shuttleworth C. A., Kielty C. M. Proteomic analysis of fibrillin-rich microfibrils. Proteomics. 2006;6:111–122. - PubMed
-
- Carta L., Pereira L., Arteaga-Solis E., Lee-Arteaga S. Y., Lenart B., Starcher B., Merkel C. A., Sukoyan M., Kerkis A., Hazeki N., Keene D. R., Sakai L. Y., Ramirez F. Fibrillins 1 and 2 perform partially overlapping functions during aortic development. J. Biol. Chem. 2006;281:8016–8023. - PMC - PubMed
-
- Charbonneau N. L., Dzamba B. J., Ono R. N., Keene D. R., Corson G. M., Reinhardt D. P., Sakai L. Y. Fibrillins can co-assemble in fibrils, but fibrillin fibril composition displays cell-specific differences. J. Biol. Chem. 2003;278:2740–2749. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

