Inhibitory effect of matrine on blood-brain barrier disruption for the treatment of experimental autoimmune encephalomyelitis
- PMID: 24194630
- PMCID: PMC3781841
- DOI: 10.1155/2013/736085
Inhibitory effect of matrine on blood-brain barrier disruption for the treatment of experimental autoimmune encephalomyelitis
Retraction in
-
RETRACTION: Inhibitory Effect of Matrine on Blood-Brain Barrier Disruption for the Treatment of Experimental Autoimmune Encephalomyelitis.Mediators Inflamm. 2025 May 29;2025:9829464. doi: 10.1155/mi/9829464. eCollection 2025. Mediators Inflamm. 2025. PMID: 40476275 Free PMC article.
Abstract
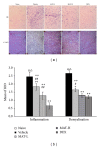
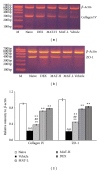
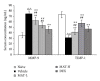
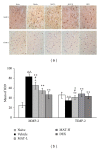
Dysfunction of the blood-brain barrier (BBB) is a primary characteristic of experimental autoimmune encephalomyelitis (EAE), an experimental model of multiple sclerosis (MS). Matrine (MAT), a quinolizidine alkaloid derived from the herb Radix Sophorae Flave, has been recently found to suppress clinical EAE and CNS inflammation. However, whether this effect of MAT is through protecting the integrity and function of the BBB is not known. In the present study, we show that MAT treatment had a therapeutic effect comparable to dexamethasone (DEX) in EAE rats, with reduced Evans Blue extravasation, increased expression of collagen IV, the major component of the basement membrane, and the structure of tight junction (TJ) adaptor protein Zonula occludens-1 (ZO-1). Furthermore, MAT treatment attenuated expression of matrix metalloproteinase-9 and -2 (MMP-9/-2), while it increased the expression of tissue inhibitors of metalloproteinase-1 and -2 (TIMP-1/-2). Our findings demonstrate that MAT reduces BBB leakage by strengthening basement membrane, inhibiting activities of MMP-2 and -9, and upregulating their inhibitors. Taken together, our results identify a novel mechanism underlying the effect of MAT, a natural compound that could be a novel therapy for MS.
Figures


Similar articles
-
Matrine protects neuro-axon from CNS inflammation-induced injury.Exp Mol Pathol. 2015 Feb;98(1):124-30. doi: 10.1016/j.yexmp.2015.01.001. Epub 2015 Jan 7. Exp Mol Pathol. 2015. Retraction in: Exp Mol Pathol. 2025 Jun;142:104968. doi: 10.1016/j.yexmp.2025.104968. PMID: 25576296 Retracted.
-
Matrine downregulates IL-33/ST2 expression in the central nervous system of rats with experimental autoimmune encephalomyelitis.Immunol Lett. 2016 Oct;178:97-104. doi: 10.1016/j.imlet.2016.08.007. Epub 2016 Aug 22. Immunol Lett. 2016. PMID: 27562326
-
Matrine treatment induced an A2 astrocyte phenotype and protected the blood-brain barrier in CNS autoimmunity.J Chem Neuroanat. 2021 Nov;117:102004. doi: 10.1016/j.jchemneu.2021.102004. Epub 2021 Jul 17. J Chem Neuroanat. 2021. PMID: 34280490
-
Upregulation of immunomodulatory molecules by matrine treatment in experimental autoimmune encephalomyelitis.Exp Mol Pathol. 2014 Dec;97(3):470-6. doi: 10.1016/j.yexmp.2014.10.004. Epub 2014 Oct 7. Exp Mol Pathol. 2014. PMID: 25303900
-
Matrine suppresses production of IL-23/IL-17 and ameliorates experimental autoimmune encephalomyelitis.Am J Chin Med. 2011;39(5):933-41. doi: 10.1142/S0192415X11009317. Am J Chin Med. 2011. PMID: 21905283
Cited by
-
Matrine treatment reduces retinal ganglion cell apoptosis in experimental optic neuritis.Sci Rep. 2021 May 4;11(1):9520. doi: 10.1038/s41598-021-89086-7. Sci Rep. 2021. PMID: 33947942 Free PMC article.
-
Calcitriol Prevents Neuroinflammation and Reduces Blood-Brain Barrier Disruption and Local Macrophage/Microglia Activation.Front Pharmacol. 2020 Mar 12;11:161. doi: 10.3389/fphar.2020.00161. eCollection 2020. Front Pharmacol. 2020. PMID: 32226379 Free PMC article.
-
Delivery of therapeutic peptides and proteins to the CNS.Adv Pharmacol. 2014;71:277-99. doi: 10.1016/bs.apha.2014.06.004. Epub 2014 Aug 22. Adv Pharmacol. 2014. PMID: 25307220 Free PMC article. Review.
-
Illumination of Molecular Pathways in Multiple Sclerosis Lesions and the Immune Mechanism of Matrine Treatment in EAE, a Mouse Model of MS.Front Immunol. 2021 Apr 12;12:640778. doi: 10.3389/fimmu.2021.640778. eCollection 2021. Front Immunol. 2021. PMID: 33912166 Free PMC article.
-
Resveratrol defends blood-brain barrier integrity in experimental autoimmune encephalomyelitis mice.J Neurophysiol. 2016 Nov 1;116(5):2173-2179. doi: 10.1152/jn.00510.2016. Epub 2016 Aug 17. J Neurophysiol. 2016. PMID: 27535376 Free PMC article.
References
-
- Rommer PS, Stüve O. Management of secondary progressive multiple sclerosis: prophy- lactic treatment-past, present, and future aspect. Current Treatment Options in Neurology. 2013;15(3):241–258. - PubMed
-
- Lassmann H. Axonal and neuronal pathology in multiple sclerosis: what have we learnt from animal models. Experimental Neurology. 2010;225(1):2–8. - PubMed
-
- Sorokin L. The impact of the extracellular matrix on inflammation. Nature Reviews Immunology. 2010;10(10):712–723. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

