Sodium channel SCN8A (Nav1.6): properties and de novo mutations in epileptic encephalopathy and intellectual disability
- PMID: 24194747
- PMCID: PMC3809569
- DOI: 10.3389/fgene.2013.00213
Sodium channel SCN8A (Nav1.6): properties and de novo mutations in epileptic encephalopathy and intellectual disability
Abstract
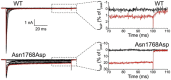
The sodium channel Nav1.6, encoded by the gene SCN8A, is one of the major voltage-gated channels in human brain. The sequences of sodium channels have been highly conserved during evolution, and minor changes in biophysical properties can have a major impact in vivo. Insight into the role of Nav1.6 has come from analysis of spontaneous and induced mutations of mouse Scn8a during the past 18 years. Only within the past year has the role of SCN8A in human disease become apparent from whole exome and genome sequences of patients with sporadic disease. Unique features of Nav1.6 include its contribution to persistent current, resurgent current, repetitive neuronal firing, and subcellular localization at the axon initial segment (AIS) and nodes of Ranvier. Loss of Nav1.6 activity results in reduced neuronal excitability, while gain-of-function mutations can increase neuronal excitability. Mouse Scn8a (med) mutants exhibit movement disorders including ataxia, tremor and dystonia. Thus far, more than ten human de novo mutations have been identified in patients with two types of disorders, epileptic encephalopathy and intellectual disability. We review these human mutations as well as the unique features of Nav1.6 that contribute to its role in determining neuronal excitability in vivo. A supplemental figure illustrating the positions of amino acid residues within the four domains and 24 transmembrane segments of Nav1.6 is provided to facilitate the location of novel mutations within the channel protein.
Keywords: Nav1.6; SCN8A; epilepsy; exomes; genetics; intellectual disability; neurogenetics; voltage-gated sodium channels.
Figures


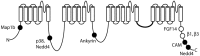
Similar articles
-
Sodium Channelopathies in Human and Animal Models of Epilepsy and Neurodevelopmental Disorders.In: Noebels JL, Avoli M, Rogawski MA, Vezzani A, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 5th edition. New York: Oxford University Press; 2024. Chapter 44. In: Noebels JL, Avoli M, Rogawski MA, Vezzani A, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 5th edition. New York: Oxford University Press; 2024. Chapter 44. PMID: 39637100 Free Books & Documents. Review.
-
Single amino acid deletion in transmembrane segment D4S6 of sodium channel Scn8a (Nav1.6) in a mouse mutant with a chronic movement disorder.Neurobiol Dis. 2016 May;89:36-45. doi: 10.1016/j.nbd.2016.01.018. Epub 2016 Jan 22. Neurobiol Dis. 2016. PMID: 26807988 Free PMC article.
-
Prax330 reduces persistent and resurgent sodium channel currents and neuronal hyperexcitability of subiculum neurons in a mouse model of SCN8A epileptic encephalopathy.Neuropharmacology. 2019 Nov 1;158:107699. doi: 10.1016/j.neuropharm.2019.107699. Epub 2019 Jul 3. Neuropharmacology. 2019. PMID: 31278928 Free PMC article.
-
Aberrant Sodium Channel Currents and Hyperexcitability of Medial Entorhinal Cortex Neurons in a Mouse Model of SCN8A Encephalopathy.J Neurosci. 2017 Aug 9;37(32):7643-7655. doi: 10.1523/JNEUROSCI.2709-16.2017. Epub 2017 Jul 4. J Neurosci. 2017. PMID: 28676574 Free PMC article.
-
SCN8A encephalopathy: Mechanisms and models.Epilepsia. 2019 Dec;60 Suppl 3(Suppl 3):S86-S91. doi: 10.1111/epi.14703. Epilepsia. 2019. PMID: 31904118 Free PMC article. Review.
Cited by
-
Gene mutations in comorbidity of epilepsy and arrhythmia.J Neurol. 2023 Mar;270(3):1229-1248. doi: 10.1007/s00415-022-11430-2. Epub 2022 Nov 14. J Neurol. 2023. PMID: 36376730 Review.
-
CaMKII enhances voltage-gated sodium channel Nav1.6 activity and neuronal excitability.J Biol Chem. 2020 Aug 14;295(33):11845-11865. doi: 10.1074/jbc.RA120.014062. Epub 2020 Jul 1. J Biol Chem. 2020. PMID: 32611770 Free PMC article.
-
Inhibitory effects of cannabidiol on voltage-dependent sodium currents.J Biol Chem. 2018 Oct 26;293(43):16546-16558. doi: 10.1074/jbc.RA118.004929. Epub 2018 Sep 14. J Biol Chem. 2018. PMID: 30219789 Free PMC article.
-
Whole-Exome Sequencing Implicates SCN2A in Episodic Ataxia, but Multiple Ion Channel Variants May Contribute to Phenotypic Complexity.Int J Mol Sci. 2018 Oct 11;19(10):3113. doi: 10.3390/ijms19103113. Int J Mol Sci. 2018. PMID: 30314295 Free PMC article.
-
Modeling human epilepsy by TALEN targeting of mouse sodium channel Scn8a.Genesis. 2014 Feb;52(2):141-8. doi: 10.1002/dvg.22731. Epub 2013 Dec 12. Genesis. 2014. PMID: 24288358 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

