A full GMP process to select and amplify epitope-specific T lymphocytes for adoptive immunotherapy of metastatic melanoma
- PMID: 24194775
- PMCID: PMC3806119
- DOI: 10.1155/2013/932318
A full GMP process to select and amplify epitope-specific T lymphocytes for adoptive immunotherapy of metastatic melanoma
Abstract
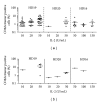
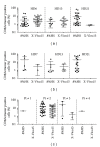
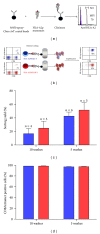
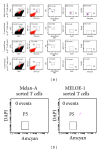
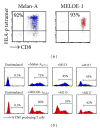
A number of trials of adoptive transfer of tumor-specific T lymphocytes have been performed in the last 20 years in metastatic melanoma, with increasingly encouraging results as the relevant melanoma antigens were identified and the purity/specificity of injected T cells improved. We have previously described a sorting method of epitope-specific T lymphocytes that uses magnetic beads coated with HLA/peptide complexes and we suggested that this method could be applied to a clinical setting. In the present work, we provide a detailed description of the whole GMP process of sorting and amplification of clinical grade T cells specific for the melanoma antigens Melan-A and MELOE-1. All the reagents used in this process including the sorting reagent were produced in GMP conditions and we document the optimization of the different steps of the process such as peptide stimulation, sorting, and amplification. The optimized procedure, validated in 3 blank runs in a clinical setting, allowed the production of at least 10⁸ pure (>90%) Melan-A- and MELOE-1-specific T cells within 28 days starting with 100 mL of blood from metastatic melanoma patients. This GMP process is thus ready to be used in an upcoming phase I/II clinical trial on metastatic melanoma patients.
Figures

Similar articles
-
Survival and tumor localization of adoptively transferred Melan-A-specific T cells in melanoma patients.J Immunol. 2003 Feb 15;170(4):2161-9. doi: 10.4049/jimmunol.170.4.2161. J Immunol. 2003. PMID: 12574389 Clinical Trial.
-
TCR Analyses of Two Vast and Shared Melanoma Antigen-Specific T Cell Repertoires: Common and Specific Features.Front Immunol. 2018 Aug 30;9:1962. doi: 10.3389/fimmu.2018.01962. eCollection 2018. Front Immunol. 2018. PMID: 30214446 Free PMC article. Clinical Trial.
-
Phase I/II clinical trial of adoptive cell transfer of sorted specific T cells for metastatic melanoma patients.Cancer Immunol Immunother. 2021 Oct;70(10):3015-3030. doi: 10.1007/s00262-021-02961-0. Epub 2021 Jun 12. Cancer Immunol Immunother. 2021. PMID: 34120214 Free PMC article. Clinical Trial.
-
Antigenicity and immunogenicity of Melan-A/MART-1 derived peptides as targets for tumor reactive CTL in human melanoma.Immunol Rev. 2002 Oct;188:81-96. doi: 10.1034/j.1600-065x.2002.18808.x. Immunol Rev. 2002. PMID: 12445283 Review.
-
Recognition of melanoma-derived antigens by CTL: possible mechanisms involved in down-regulating anti-tumor T-cell reactivity.Crit Rev Immunol. 1998;18(1-2):55-63. doi: 10.1615/critrevimmunol.v18.i1-2.70. Crit Rev Immunol. 1998. PMID: 9419448 Review.
Cited by
-
Manufacture of tumor- and virus-specific T lymphocytes for adoptive cell therapies.Cancer Gene Ther. 2015 Mar;22(2):85-94. doi: 10.1038/cgt.2014.81. Epub 2015 Feb 27. Cancer Gene Ther. 2015. PMID: 25721207 Free PMC article. Review.
-
PD-1 expression on tumor-specific T cells: Friend or foe for immunotherapy?Oncoimmunology. 2017 Sep 14;7(1):e1364828. doi: 10.1080/2162402X.2017.1364828. eCollection 2017. Oncoimmunology. 2017. PMID: 29296515 Free PMC article. Review.
-
Adoptive T cell therapy combined with intralesional administrations of TG1042 (adenovirus expressing interferon-γ) in metastatic melanoma patients.Cancer Immunol Immunother. 2015 Jul;64(7):805-15. doi: 10.1007/s00262-015-1691-7. Epub 2015 Apr 7. Cancer Immunol Immunother. 2015. PMID: 25846669 Free PMC article. Clinical Trial.
-
Trial Watch: Adoptive cell transfer for anticancer immunotherapy.Oncoimmunology. 2014 May 1;3:e28344. doi: 10.4161/onci.28344. eCollection 2014. Oncoimmunology. 2014. PMID: 25050207 Free PMC article. Review.
-
The interplay between membrane topology and mechanical forces in regulating T cell receptor activity.Commun Biol. 2022 Jan 11;5(1):40. doi: 10.1038/s42003-021-02995-1. Commun Biol. 2022. PMID: 35017678 Free PMC article. Review.
References
-
- Rooney CM, Roskrow MA, Suzuki N, Ng CYC, Brenner MK, Heslop H. Treatment of relapsed Hodgkin’s disease using EBV-specific cytotoxic T cells. Annals of Oncology. 1998;9(5):S129–S132. - PubMed
-
- Khammari A, Labarrière N, Vignard V, et al. Treatment of metastatic melanoma with autologous Melan-A/MART-1-specific cytotoxic T lymphocyte clones. The Journal of Investigative Dermatology. 2009;129(12):2835–2842. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

