Overtreatment of COPD with inhaled corticosteroids--implications for safety and costs: cross-sectional observational study
- PMID: 24194824
- PMCID: PMC3806778
- DOI: 10.1371/journal.pone.0075221
Overtreatment of COPD with inhaled corticosteroids--implications for safety and costs: cross-sectional observational study
Abstract
Introduction: Combined inhaled long-acting beta-agonists and corticosteroids (LABA+ICS) are costly. They are recommended in severe or very severe chronic obstructive pulmonary disease (COPD). They should not be prescribed in mild or moderate disease. In COPD ICS are associated with side-effects including risk of pneumonia. We quantified appropriateness of prescribing and examined the risks and costs associated with overuse.
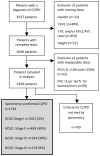
Methods: Data were extracted from the electronic and paper records of 41 London general practices (population 310,775) including spirometry, medications and exacerbations. We classified severity, assessed appropriateness of prescribing using the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines for 2009, and performed a sensitivity analysis using the broader recommendations of the 2011 revision.
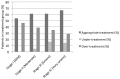
Results: 3537 patients had a diagnosis of COPD. Spirometry was recorded for 2458(69%). 709(29%) did not meet GOLD criteria. 1749(49%) with confirmed COPD were analysed: 8.6% under-treated, 38% over-treated. Over-prescription of ICS in GOLD stage I or II (n=403, 38%) and in GOLD III or IV without exacerbations (n=231, 33.6%) was common. An estimated 12 cases (95%CI 7-19) annually of serious pneumonia were likely among 897 inappropriately treated. 535 cases of overtreatment involved LABA+ICS with a mean per patient cost of £553.56/year (€650.03). Using the broader indications for ICS in the 2011 revised GOLD guideline 25% were still classified as over-treated. The estimated risk of 15 cases of pneumonia (95%CI 8-22) in 1074 patients currently receiving ICS would rise by 20% to 18 (95%CI 9.8-26.7) in 1305 patients prescribed ICS if all with GOLD grade 3 and 4 received LABA+ICS.
Conclusion: Over-prescription of ICS in confirmed COPD was widespread with considerable potential for harm. In COPD where treatment is often escalated in the hope of easing the burden of disease clinicians should consider both the risks and benefits of treatment and the costs where the benefits are unproven.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical