The immunological and virological consequences of planned treatment interruptions in children with HIV infection
- PMID: 24194841
- PMCID: PMC3806774
- DOI: 10.1371/journal.pone.0076582
The immunological and virological consequences of planned treatment interruptions in children with HIV infection
Abstract
Objectives: To evaluate the immunological and viral consequences of planned treatment interruptions (PTI) in children with HIV.
Design: This was an immunological and virological sub-study of the Paediatric European Network for Treatment of AIDS (PENTA) 11 trial, which compared CD4-guided PTI of antiretroviral therapy (ART) with continuous therapy (CT) in children.
Methods: HIV-1 RNA and lymphocyte subsets, including CD4 and CD8 cells, were quantified on fresh samples collected during the study; CD45RA, CD45RO and CD31 subpopulations were evaluated in some centres. For 36 (18 PTI, 18 CT) children, immunophenotyping was performed and cell-associated HIV-1 DNA analysed on stored samples to 48 weeks.
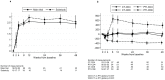
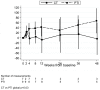
Results: In the PTI group, CD4 cell count fell rapidly in the first 12 weeks off ART, with decreases in both naïve and memory cells. However, the proportion of CD4 cells expressing CD45RA and CD45RO remained constant in both groups. The increase in CD8 cells in the first 12 weeks off ART in the PTI group was predominantly due to increases in RO-expressing cells. PTI was associated with a rapid and sustained increase in CD4 cells expressing Ki67 and HLA-DR, and increased levels of HIV-1 DNA.
Conclusions: PTI in children is associated with rapid changes in CD4 and CD8 cells, likely due to increased cell turnover and immune activation. However, children off treatment may be able to maintain stable levels of naïve CD4 cells, at least in proportion to the memory cell pool, which may in part explain the observed excellent CD4 cell recovery with re-introduction of ART.
Conflict of interest statement
Figures



References
-
- Paediatric European Network for Treatment of AIDS (2010) Response to planned treatment interruptions in HIV infection varies across childhood. AIDS 24: 231–241. - PubMed
-
- Bunupuradah T, Duong T, Compagnucci A, McMaster P, Bernardi S, et al, on behalf of the PENTA 11 Extension Study Group (2013) Outcomes after reinitiating antiretroviral therapy in children randomized to planned treatment interruptions in the PENTA 11 Study. AIDS 27: 579–589. - PubMed
-
- Ananworanich J, Gayet-Ageron A, Le Braz M, Prasithsirikul W, Chetchotisakd P, et al. (2006) CD4-guided scheduled treatment interruptions compared with continuous therapy for patients infected with HIV-1: results of the Staccato randomised trial. Lancet 368: 459–465. - PubMed
-
- Danel C, Moh R, Minga A, Anzian A, Ba-Gomis O, et al. (2006) CD4-guided structured antiretroviral treatment interruption strategy in HIV-infected adults in west Africa (Trivacan ANRS 1269 trial): a randomised trial. Lancet 367: 1981–9. - PubMed
-
- Strategies for Management of Antiretroviral Therapy (SMART) Study Group, El-Sadr WM, Lundgren JD, Neaton JD, Gordin F, et al (2006) CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med 355: 2283–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

