Discovery of p1736, a novel antidiabetic compound that improves peripheral insulin sensitivity in mice models
- PMID: 24194903
- PMCID: PMC3806773
- DOI: 10.1371/journal.pone.0077946
Discovery of p1736, a novel antidiabetic compound that improves peripheral insulin sensitivity in mice models
Erratum in
- PLoS One. 2014;9(2):e91390. Mutt, Shivaprakash [corrected to Mutt, Shivaprakash Jagalur]
- PLoS One. 2014;9(7):e103474
Abstract
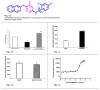
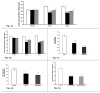
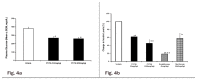
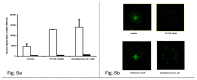
Insulin resistance is a characteristic feature of Type 2 diabetes. Insulin resistance has also been implicated in the pathogenesis of cardiovascular disease. Currently used thiazolidinedione (TZD) insulin sensitizers although effective, have adverse side effects of weight gain, fluid retention and heart failure. Using fat cell-based phenotypic drug discovery approach we identified P1736, a novel antidiabetic molecule that has completed Phase II clinical trials. The present study evaluated the in vitro and in vivo pharmacological properties of P1736. P1736 is a non-TZD and it did not activate human PPAR(Peroxisome Proliferator Activated Receptor Gamma )receptors. P1736 caused dose dependent increase in glucose uptake (EC50-400 nM) in the insulin resistant 3T3 adipocytes. The compound (10 µM) induced translocation of GLUT-4 (Glucose Transporter type 4) transporters in these adipocytes while metformin (1.0mM) was inactive. In diabetic db/db mice, P1736 (150 mg/kg) was more efficacious than metformin in lowering plasma glucose (35% vs 25%) and triglyceride levels (38% vs 31%). P1736 tested at 5mg/kg, twice daily doses, reduced glucose by 41% and triglycerides by 32%, in db/db mice. These effects were not associated with adverse effects on body weight or liver function. Rosiglitazone (5mg/kg, twice daily) caused 60% and 40 % decreases in glucose and triglyceride levels, respectively. However, rosiglitazone induced 13% weight gain (p<0.05) in db/db mice. P1736 was also efficacious in ob/ob mice wherein 30-35% decrease in glucose and significant improvement in hyperinsulinemia were observed. Administration of P1736 to ob/ob mice resulted in 70% increase in glucose uptake in soleus muscles while metformin caused 38% increase. P1736 exhibited excellent safety profile and was weight neutral in all preclinical models of diabetes. Thus, P1736 with its unique pharmacology coupled with PPAR- independent mode of action could represent an alternative option in the management of insulin resistant Type 2 diabetic patients.
Conflict of interest statement
Figures

Similar articles
-
18F9 (4-(3,6-bis (ethoxycarbonyl)-4,5,6,7-tetrahydrothieno (2,3-c) pyridin-2-ylamino)-4-oxobutanoic acid) enhances insulin-mediated glucose uptake in vitro and exhibits antidiabetic activity in vivo in db/db mice.Metabolism. 2009 Oct;58(10):1503-16. doi: 10.1016/j.metabol.2009.04.036. Epub 2009 Jul 15. Metabolism. 2009. PMID: 19608207
-
KDT501, a derivative from hops, normalizes glucose metabolism and body weight in rodent models of diabetes.PLoS One. 2014 Jan 30;9(1):e87848. doi: 10.1371/journal.pone.0087848. eCollection 2014. PLoS One. 2014. PMID: 24498211 Free PMC article.
-
T2384, a novel antidiabetic agent with unique peroxisome proliferator-activated receptor gamma binding properties.J Biol Chem. 2008 Apr 4;283(14):9168-76. doi: 10.1074/jbc.M800104200. Epub 2008 Feb 7. J Biol Chem. 2008. PMID: 18263587
-
[Thiazolidinediones in type 2 diabetes. Role of peroxisome proliferator-activated receptor gamma (PPARgamma)].Ann Endocrinol (Paris). 2002 Dec;63(6 Pt 1):511-23. Ann Endocrinol (Paris). 2002. PMID: 12527853 Review. French.
-
Oral antidiabetic agents: current role in type 2 diabetes mellitus.Drugs. 2005;65(3):385-411. doi: 10.2165/00003495-200565030-00005. Drugs. 2005. PMID: 15669880 Review.
Cited by
-
Metformin ameliorates hepatic steatosis and inflammation without altering adipose phenotype in diet-induced obesity.PLoS One. 2014 Mar 17;9(3):e91111. doi: 10.1371/journal.pone.0091111. eCollection 2014. PLoS One. 2014. PMID: 24638078 Free PMC article.
-
Heterozygous deletion of Seipin in islet beta cells of male mice has an impact on insulin synthesis and secretion through reduced PPARγ expression.Diabetologia. 2020 Feb;63(2):338-350. doi: 10.1007/s00125-019-05038-x. Epub 2019 Nov 27. Diabetologia. 2020. PMID: 31776610
-
The Drug Discovery and Development Industry in India-Two Decades of Proprietary Small-Molecule R&D.ChemMedChem. 2017 Jun 7;12(11):786-818. doi: 10.1002/cmdc.201700043. Epub 2017 Jun 1. ChemMedChem. 2017. PMID: 28464443 Free PMC article. Review.
-
A role for PFKFB3/iPFK2 in metformin suppression of adipocyte inflammatory responses.J Mol Endocrinol. 2017 Jul;59(1):49-59. doi: 10.1530/JME-17-0066. J Mol Endocrinol. 2017. PMID: 28559290 Free PMC article.
-
Glucose dysregulation and response to common anti-diabetic agents in the FATZO/Pco mouse.PLoS One. 2017 Jun 22;12(6):e0179856. doi: 10.1371/journal.pone.0179856. eCollection 2017. PLoS One. 2017. PMID: 28640857 Free PMC article.
References
-
- Singh S, Loke YK (2008) The safety of rosiglitazone in the treatment of type 2 diabetes. Expert.Opin. Drug Saf 7: 579-585. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

