Maternal transfer of the cyanobacterial neurotoxin β-N-methylamino-L-alanine (BMAA) via milk to suckling offspring
- PMID: 24194910
- PMCID: PMC3806833
- DOI: 10.1371/journal.pone.0078133
Maternal transfer of the cyanobacterial neurotoxin β-N-methylamino-L-alanine (BMAA) via milk to suckling offspring
Erratum in
-
Correction: Maternal Transfer of the Cyanobacterial Neurotoxin β-N-Methylamino-L-Alanine (BMAA) via Milk to Suckling Offspring.PLoS One. 2015 Jul 14;10(7):e0133110. doi: 10.1371/journal.pone.0133110. eCollection 2015. PLoS One. 2015. PMID: 26172384 Free PMC article. No abstract available.
Abstract
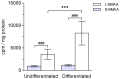
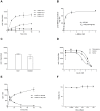
The cyanobacterial neurotoxin β-N-methylamino-L-alanine (BMAA) has been implicated in the etiology of neurodegenerative disease and proposed to be biomagnified in terrestrial and aquatic food chains. We have previously shown that the neonatal period in rats, which in humans corresponds to the last trimester of pregnancy and the first few years of age, is a particularly sensitive period for exposure to BMAA. The present study aimed to examine the secretion of (14)C-labeled L- and D-BMAA into milk in lactating mice and the subsequent transfer of BMAA into the developing brain. The results suggest that secretion into milk is an important elimination pathway of BMAA in lactating mothers and an efficient exposure route predominantly for L-BMAA but also for D-BMAA in suckling mice. Following secretion of [(14)C]L-BMAA into milk, the levels of [(14)C]L-BMAA in the brains of the suckling neonatal mice significantly exceeded the levels in the maternal brains. In vitro studies using the mouse mammary epithelial HC11 cell line confirmed a more efficient influx and efflux of L-BMAA than of D-BMAA in cells, suggesting enantiomer-selective transport. Competition experiments with other amino acids and a low sodium dependency of the influx suggests that the amino acid transporters LAT1 and LAT2 are involved in the transport of L-BMAA into milk. Given the persistent neurodevelopmental toxicity following injection of L-BMAA to neonatal rodent pups, the current results highlight the need to determine whether BMAA is enriched mother's and cow's milk.
Conflict of interest statement
Figures
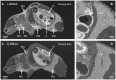
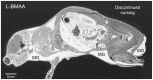
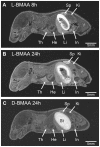
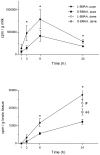
References
-
- Spencer PS, Hugon J, Ludolph A, Nunn PB, Ross SM, et al. (1987) Discovery and partial characterization of primate motor-system toxins. Ciba Found Symp 126: 221–238. - PubMed
-
- Murch SJ, Cox PA, Banack SA, Steele JC, Sacks OW (2004) Occurrence of beta-methylamino-l-alanine (BMAA) in ALS/PDC patients from Guam. Acta Neurol Scand 110: 267–269. - PubMed
-
- Pablo J, Banack SA, Cox PA, Johnson TE, Papapetropoulos S, et al. (2009) Cyanobacterial neurotoxin BMAA in ALS and Alzheimer's disease. Acta Neurol Scand 120: 216–225. - PubMed
-
- Bradley WG, Borenstein AR, Nelson LM, Codd GA, Rosen BH, et al. (2013) Is exposure to cyanobacteria an environmental risk factor for amyotrophic lateral sclerosis and other neurodegenerative diseases? Amyotroph Lateral Scler Frontotemporal Degener 14: 325–333. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

