Local IL-17A potentiates early neutrophil recruitment to the respiratory tract during severe RSV infection
- PMID: 24194936
- PMCID: PMC3806820
- DOI: 10.1371/journal.pone.0078461
Local IL-17A potentiates early neutrophil recruitment to the respiratory tract during severe RSV infection
Abstract
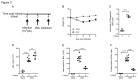
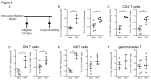
Respiratory syncytial virus (RSV) bronchiolitis triggers a strong innate immune response characterized by excessive neutrophil infiltration which contributes to RSV induced pathology. The cytokine IL-17A enhances neutrophil infiltration into virus infected lungs. IL-17A is however best known as an effector of adaptive immune responses. The role of IL-17A in early immune modulation in RSV infection is unknown. We aimed to elucidate whether local IL-17A facilitates the innate neutrophil infiltration into RSV infected lungs prior to adaptive immunity. To this end, we studied IL-17A production in newborns that were hospitalized for severe RSV bronchiolitis. In tracheal aspirates we measured IL-17A concentration and neutrophil counts. We utilized cultured human epithelial cells to test if IL-17A regulates RSV infection-induced IL-8 release as mediator of neutrophil recruitment. In mice we investigated the cell types that are responsible for early innate IL-17A production during RSV infection. Using IL-17A neutralizing antibodies we tested if IL-17A is responsible for innate neutrophil infiltration in mice. Our data show that increased IL-17A production in newborn RSV patient lungs correlates with subsequent neutrophil counts recruited to the lungs. IL-17A potentiates RSV-induced production of the neutrophil-attracting chemokine IL-8 by airway epithelial cells in vitro. Various lung-resident lymphocytes produced IL-17A during early RSV infection in Balb/c mice, of which a local population of CD4 T cells stood out as the predominant RSV-induced cell type. By removing IL-17A during early RSV infection in mice we showed that IL-17A is responsible for enhanced innate neutrophil infiltration in vivo. Using patient material, in vitro studies, and an animal model of RSV infection, we thus show that early local IL-17A production in the airways during RSV bronchiolitis facilitates neutrophil recruitment with pathologic consequences to infant lungs.
Conflict of interest statement
Figures



Similar articles
-
Respiratory Syncytial virus infection compromises asthma tolerance by recruiting interleukin-17A-producing cells via CCR6-CCL20 signaling.Mol Immunol. 2017 Aug;88:45-57. doi: 10.1016/j.molimm.2017.05.017. Epub 2017 Jun 7. Mol Immunol. 2017. PMID: 28599122
-
IL-13 regulates Th17 secretion of IL-17A in an IL-10-dependent manner.J Immunol. 2012 Feb 1;188(3):1027-35. doi: 10.4049/jimmunol.1102216. Epub 2011 Dec 30. J Immunol. 2012. PMID: 22210911 Free PMC article.
-
Innate cellular sources of interleukin-17A regulate macrophage accumulation in cigarette- smoke-induced lung inflammation in mice.Clin Sci (Lond). 2015 Nov;129(9):785-96. doi: 10.1042/CS20140703. Epub 2015 Jul 1. Clin Sci (Lond). 2015. PMID: 26201093 Free PMC article.
-
Respiratory syncytial virus (RSV) evades the human adaptive immune system by skewing the Th1/Th2 cytokine balance toward increased levels of Th2 cytokines and IgE, markers of allergy--a review.Virus Genes. 2006 Oct;33(2):235-52. doi: 10.1007/s11262-006-0064-x. Virus Genes. 2006. PMID: 16972040 Review.
-
Neutrophil-endothelial interactions in respiratory syncytial virus bronchiolitis: An understudied aspect with a potential for prediction of severity of disease.J Clin Virol. 2020 Feb;123:104258. doi: 10.1016/j.jcv.2019.104258. Epub 2019 Dec 31. J Clin Virol. 2020. PMID: 31931445 Review.
Cited by
-
Inflammation as a Regulator of the Airway Surface Liquid pH in Cystic Fibrosis.Cells. 2023 Apr 7;12(8):1104. doi: 10.3390/cells12081104. Cells. 2023. PMID: 37190013 Free PMC article. Review.
-
TNFα and IL-17 alkalinize airway surface liquid through CFTR and pendrin.Am J Physiol Cell Physiol. 2020 Aug 1;319(2):C331-C344. doi: 10.1152/ajpcell.00112.2020. Epub 2020 May 20. Am J Physiol Cell Physiol. 2020. PMID: 32432926 Free PMC article.
-
Inhibition of autophagy promotes human RSV NS1-induced inflammation and apoptosis in vitro.Exp Ther Med. 2021 Oct;22(4):1054. doi: 10.3892/etm.2021.10488. Epub 2021 Jul 23. Exp Ther Med. 2021. PMID: 34434268 Free PMC article.
-
Yin and yang of interleukin-17 in host immunity to infection.F1000Res. 2017 May 23;6:741. doi: 10.12688/f1000research.10862.1. eCollection 2017. F1000Res. 2017. PMID: 28713557 Free PMC article. Review.
-
Na+/H+ Exchanger Regulatory Factor 1 Mediates the Pathogenesis of Airway Inflammation in a Murine Model of House Dust Mite-Induced Asthma.J Immunol. 2021 May 15;206(10):2301-2311. doi: 10.4049/jimmunol.2001199. Epub 2021 May 5. J Immunol. 2021. PMID: 33952618 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

