Energetic contributions to channel gating of residues in the muscle nicotinic receptor β1 subunit
- PMID: 24194945
- PMCID: PMC3806828
- DOI: 10.1371/journal.pone.0078539
Energetic contributions to channel gating of residues in the muscle nicotinic receptor β1 subunit
Abstract
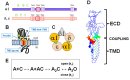
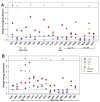
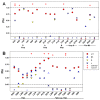
In the pentameric ligand-gated ion channel family, transmitter binds in the extracellular domain and conformational changes result in channel opening in the transmembrane domain. In the muscle nicotinic receptor and other heteromeric members of the family one subunit does not contribute to the canonical agonist binding site for transmitter. A fundamental question is whether conformational changes occur in this subunit. We used records of single channel activity and rate-equilibrium free energy relationships to examine the β1 (non-ACh-binding) subunit of the muscle nicotinic receptor. Mutations to residues in the extracellular domain have minimal effects on the gating equilibrium constant. Positions in the channel lining (M2 transmembrane) domain contribute strongly and relatively late during gating. Positions thought to be important in other subunits in coupling the transmitter-binding to the channel domains have minimal effects on gating. We conclude that the conformational changes involved in channel gating propagate from the binding-site to the channel in the ACh-binding subunits and subsequently spread to the non-binding subunit.
Conflict of interest statement
Figures




Similar articles
-
Initial coupling of binding to gating mediated by conserved residues in the muscle nicotinic receptor.J Gen Physiol. 2005 Jul;126(1):23-39. doi: 10.1085/jgp.200509283. Epub 2005 Jun 13. J Gen Physiol. 2005. PMID: 15955875 Free PMC article.
-
A release of local subunit conformational heterogeneity underlies gating in a muscle nicotinic acetylcholine receptor.Nat Commun. 2024 Feb 27;15(1):1803. doi: 10.1038/s41467-024-46028-x. Nat Commun. 2024. PMID: 38413583 Free PMC article.
-
Subunit symmetry at the extracellular domain-transmembrane domain interface in acetylcholine receptor channel gating.J Biol Chem. 2010 Dec 10;285(50):38898-904. doi: 10.1074/jbc.M110.169110. Epub 2010 Sep 23. J Biol Chem. 2010. PMID: 20864527 Free PMC article.
-
Gating of nicotinic ACh receptors; new insights into structural transitions triggered by agonist binding that induce channel opening.J Physiol. 2007 Nov 1;584(Pt 3):727-33. doi: 10.1113/jphysiol.2007.142554. Epub 2007 Sep 6. J Physiol. 2007. PMID: 17823204 Free PMC article. Review.
-
The nicotinic receptor ligand binding domain.J Neurobiol. 2002 Dec;53(4):431-46. doi: 10.1002/neu.10139. J Neurobiol. 2002. PMID: 12436411 Review.
Cited by
-
Genome-wide association analyses identify 39 new susceptibility loci for diverticular disease.Nat Genet. 2018 Oct;50(10):1359-1365. doi: 10.1038/s41588-018-0203-z. Epub 2018 Sep 3. Nat Genet. 2018. PMID: 30177863 Free PMC article.
-
Functional anatomy of an allosteric protein.Nat Commun. 2013;4:2984. doi: 10.1038/ncomms3984. Nat Commun. 2013. PMID: 24352193 Free PMC article.
-
Activation of the Rat α1β2ε GABAA Receptor by Orthosteric and Allosteric Agonists.Biomolecules. 2022 Jun 21;12(7):868. doi: 10.3390/biom12070868. Biomolecules. 2022. PMID: 35883422 Free PMC article.
References
-
- Moroni M, Zwart R, Sher E, Cassels BK, Bermudez I (2006) alpha4beta2 nicotinic receptors with high and low acetylcholine sensitivity: pharmacology, stoichiometry, and sensitivity to long-term exposure to nicotine. Mol Pharmacol 70: 755-768. doi:10.1124/mol.106.023044. PubMed: 16720757. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

