Chronic lymphocytic leukemia patients have a preserved cytomegalovirus-specific antibody response despite progressive hypogammaglobulinemia
- PMID: 24194956
- PMCID: PMC3806856
- DOI: 10.1371/journal.pone.0078925
Chronic lymphocytic leukemia patients have a preserved cytomegalovirus-specific antibody response despite progressive hypogammaglobulinemia
Abstract
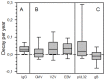
Chronic lymphocytic leukemia (CLL) is characterized by progressive hypogammaglobulinemia predisposing affected patients to a variety of infectious diseases but paradoxically not to cytomegalovirus (CMV) disease. Moreover, we found reactivity of a panel of CLL recombinant antibodies (CLL-rAbs) encoded by a germ-line allele with a single CMV protein, pUL32, despite differing antibody binding motifs. To put these findings into perspective, we studied prospectively relative frequency of viremia, kinetics of total and virus-specific IgG over time, and UL32 genetic variation in a cohort of therapy-naive patients (n=200). CMV-DNA was detected in 3% (6/200) of patients. The decay of total IgG was uniform (mean, 0.03; SD, 0.03) and correlated with that of IgG subclasses 1-4 in the paired samples available (n=64; p<0.001). Total CMV-specific IgG kinetics were more variable (mean, 0,02; SD, 0,06) and mean decay values differed significantly from those of total IgG (p=0.034). Boosts of CMV-specific antibody levels were observed in 49% (22/45) of CMV-seropositive patients. In contrast, VZV- and EBV-specific IgG levels decayed in parallel with total IgG levels (p=0.003 and p=0.001, respectively). VZV-specific IgG even became undetectable in 18% (9/50) of patients whereas CMV-specific ones remained detectable in all seropositive patients. The observed CMV-specific IgG kinetics were predicated upon the highly divergent kinetics of IgG specific for individual antigens - glycoprotein B-specific IgG were boosted in 51% and pUL32-specific IgG in 32% of patients. In conclusion, CLL patients have a preserved CMV-specific antibody response despite progressive decay of total IgG and IgG subclasses. CMV-specific IgG levels are frequently boosted in contrast to that of other herpesviruses indicative of a higher rate of CMV reactivation and antigen-presentation. In contrast to the reactivity of multiple different CLL-rAbs with pUL32, boosts of humoral immunity are triggered apparently by other CMV antigens than pUL32, like glycoprotein B.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

