The tumor targeted superantigen ABR-217620 selectively engages TRBV7-9 and exploits TCR-pMHC affinity mimicry in mediating T cell cytotoxicity
- PMID: 24194959
- PMCID: PMC3806850
- DOI: 10.1371/journal.pone.0079082
The tumor targeted superantigen ABR-217620 selectively engages TRBV7-9 and exploits TCR-pMHC affinity mimicry in mediating T cell cytotoxicity
Abstract
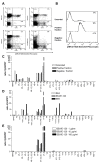
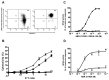
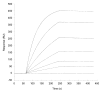
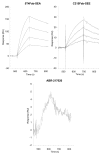
The T lymphocytes are the most important effector cells in immunotherapy of cancer. The conceptual objective for developing the tumor targeted superantigen (TTS) ABR-217620 (naptumomab estafenatox, 5T4Fab-SEA/E-120), now in phase 3 studies for advanced renal cell cancer, was to selectively coat tumor cells with cytotoxic T lymphocytes (CTL) target structures functionally similar to natural CTL pMHC target molecules. Here we present data showing that the molecular basis for the anti-tumor activity by ABR-217620 resides in the distinct interaction between the T cell receptor β variable (TRBV) 7-9 and the engineered superantigen (Sag) SEA/E-120 in the fusion protein bound to the 5T4 antigen on tumor cells. Multimeric but not monomeric ABR-217620 selectively stains TRBV7-9 expressing T lymphocytes from human peripheral blood similar to antigen specific staining of T cells with pMHC tetramers. SEA/E-120 selectively activates TRBV7-9 expressing T lymphocytes resulting in expansion of the subset. ABR-217620 selectively triggers TRBV7-9 expressing cytotoxic T lymphocytes to kill 5T4 positive tumor cells. Furthermore, ABR-217620 activates TRBV7-9 expressing T cell line cells in the presence of cell- and bead-bound 5T4 tumor antigen. Surface plasmon resonance analysis revealed that ABR-217620 binds to 5T4 with high affinity, to TRBV7-9 with low affinity and to MHC class II with very low affinity. The T lymphocyte engagement by ABR-217620 is constituted by displaying high affinity binding to the tumor cells (KD approximately 1 nM) and with the mimicry of natural productive immune TCR-pMHC contact using affinities of around 1 µM. This difference in kinetics between the two components of the ABR-217620 fusion protein will bias the binding towards the 5T4 target antigen, efficiently activating T-cells via SEA/E-120 only when presented by the tumor cells.
Conflict of interest statement
Figures



Similar articles
-
Naptumomab estafenatox: targeted immunotherapy with a novel immunotoxin.Curr Oncol Rep. 2014 Feb;16(2):370. doi: 10.1007/s11912-013-0370-0. Curr Oncol Rep. 2014. PMID: 24445502 Free PMC article. Review.
-
Naptumomab estafenatox, an engineered antibody-superantigen fusion protein with low toxicity and reduced antigenicity.J Immunother. 2010 Jun;33(5):492-9. doi: 10.1097/CJI.0b013e3181d75820. J Immunother. 2010. PMID: 20463598
-
Superantigen-staphylococcal-enterotoxin-A-dependent and antibody-targeted lysis of GD2-positive neuroblastoma cells.Cancer Immunol Immunother. 1995 Aug;41(2):129-36. doi: 10.1007/BF01527409. Cancer Immunol Immunother. 1995. PMID: 7656271 Free PMC article.
-
MHC class II-independent, Vbeta-specific activation of T cells by superantigen mutants fused to anti-tumor Fab fragments: implications for use in treatment of human colon carcinoma.Int J Mol Med. 1998 Jan;1(1):157-62. doi: 10.3892/ijmm.1.1.157. Int J Mol Med. 1998. PMID: 9852214
-
Structure-function studies of T-cell receptor-superantigen interactions.Immunol Rev. 1998 Jun;163:177-86. doi: 10.1111/j.1600-065x.1998.tb01196.x. Immunol Rev. 1998. PMID: 9700510 Review.
Cited by
-
Tumor-targeted superantigens produce curative tumor immunity with induction of memory and demonstrated antigen spreading.J Transl Med. 2023 Mar 26;21(1):222. doi: 10.1186/s12967-023-04064-z. J Transl Med. 2023. PMID: 36967382 Free PMC article.
-
Tagging staphylococcal enterotoxin B (SEB) with TGFaL3 for breast cancer therapy.Tumour Biol. 2016 Apr;37(4):5305-16. doi: 10.1007/s13277-015-4334-x. Epub 2015 Nov 11. Tumour Biol. 2016. PMID: 26561468
-
Two common structural motifs for TCR recognition by staphylococcal enterotoxins.Sci Rep. 2016 May 16;6:25796. doi: 10.1038/srep25796. Sci Rep. 2016. PMID: 27180909 Free PMC article.
-
Naptumomab estafenatox: targeted immunotherapy with a novel immunotoxin.Curr Oncol Rep. 2014 Feb;16(2):370. doi: 10.1007/s11912-013-0370-0. Curr Oncol Rep. 2014. PMID: 24445502 Free PMC article. Review.
-
Immunological response and overall survival in a subset of advanced renal cell carcinoma patients from a randomized phase 2/3 study of naptumomab estafenatox plus IFN-α versus IFN-α.Oncotarget. 2015 Feb 28;6(6):4428-39. doi: 10.18632/oncotarget.2922. Oncotarget. 2015. PMID: 25669986 Free PMC article. Clinical Trial.
References
-
- Yee C, Thompson JA, Byrd D, Riddell SR, Roche P et al. (2002) Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci U S A 99: 16168-16173. doi:10.1073/pnas.242600099. PubMed: 12427970. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

