Non-LTR retrotransposons and microsatellites: Partners in genomic variation
- PMID: 24195012
- PMCID: PMC3812793
- DOI: 10.4161/mge.25674
Non-LTR retrotransposons and microsatellites: Partners in genomic variation
Abstract
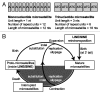
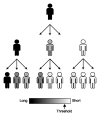
The human genome is laden with both non-LTR (long-terminal repeat) retrotransposons and microsatellite repeats. Both types of sequences are able to, either actively or passively, mutagenize the genomes of human individuals and are therefore poised to dynamically alter the human genomic landscape across generations. Non-LTR retrotransposons, such as L1 and Alu, are a major source of new microsatellites, which are born both concurrently and subsequently to L1 and Alu integration into the genome. Likewise, the mutation dynamics of microsatellite repeats have a direct impact on the fitness of their non-LTR retrotransposon parent owing to microsatellite expansion and contraction. This review explores the interactions and dynamics between non-LTR retrotransposons and microsatellites in the context of genomic variation and evolution.
Keywords: Alu; LINE-1; genomic instability; genomic variation; microsatellite; mononucleotide repeat; mutation rate; poly(A); retrotransposition; retrotransposon.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
