Identification of Bax-interacting proteins in oligodendrocyte progenitors during glutamate excitotoxicity and perinatal hypoxia-ischemia
- PMID: 24195677
- PMCID: PMC3891358
- DOI: 10.1042/AN20130027
Identification of Bax-interacting proteins in oligodendrocyte progenitors during glutamate excitotoxicity and perinatal hypoxia-ischemia
Abstract
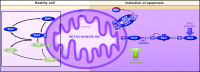
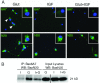
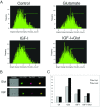
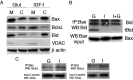
OPC (oligodendrocyte progenitor cell) death contributes significantly to the pathology and functional deficits following hypoxic-ischemic injury in the immature brain and to deficits resulting from demyelinating diseases, trauma and degenerative disorders in the adult CNS. Glutamate toxicity is a major cause of oligodendroglial death in diverse CNS disorders, and previous studies have demonstrated that AMPA/kainate receptors require the pro-apoptotic protein Bax in OPCs undergoing apoptosis. The goal of the present study was to define the pro-apoptotic and anti-apoptotic effectors that regulate Bax in healthy OPCs and after exposure to excess glutamate in vitro and following H-I (hypoxia-ischemia) in the immature rat brain. We show that Bax associates with a truncated form of Bid, a BH3-only domain protein, subsequent to glutamate treatment. Furthermore, glutamate exposure reduces Bax association with the anti-apoptotic Bcl family member, Bcl-xL. Cell fractionation studies demonstrated that both Bax and Bid translocate from the cytoplasm to mitochondria during the early stages of cell death consistent with a role for Bid as an activator, whereas Bcl-xL, which normally complexes with both Bax and Bid, disassociates from these complexes when OPCs are exposed to excess glutamate. Bax remained unactivated in the presence of insulin-like growth factor-1, and the Bcl-xL complexes were protected. Our data similarly demonstrate loss of Bcl-xL-Bax association in white matter following H-I and implicate active Bad in Bax-mediated OPC death. To identify other Bax-binding partners, we used proteomics and identified cofilin as a Bax-associated protein in OPCs. Cofilin and Bax associated in healthy OPCs, whereas the Bax-cofilin association was disrupted during glutamate-induced OPC apoptosis.
Figures


Similar articles
-
IGF-I prevents glutamate-mediated bax translocation and cytochrome C release in O4+ oligodendrocyte progenitors.Glia. 2004 Apr 15;46(2):183-94. doi: 10.1002/glia.10360. Glia. 2004. PMID: 15042585
-
Delayed IGF-1 administration rescues oligodendrocyte progenitors from glutamate-induced cell death and hypoxic-ischemic brain damage.Dev Neurosci. 2007;29(4-5):302-10. doi: 10.1159/000105471. Dev Neurosci. 2007. PMID: 17762198
-
Bax-inhibiting peptide protects glutamate-induced cerebellar granule cell death by blocking Bax translocation.Neurosci Lett. 2009 Feb 13;451(1):11-5. doi: 10.1016/j.neulet.2008.12.021. Epub 2008 Dec 24. Neurosci Lett. 2009. PMID: 19110033
-
IGF-1 protects oligodendrocyte progenitors against TNFalpha-induced damage by activation of PI3K/Akt and interruption of the mitochondrial apoptotic pathway.Glia. 2007 Aug 15;55(11):1099-107. doi: 10.1002/glia.20530. Glia. 2007. PMID: 17577243
-
Bid: a Bax-like BH3 protein.Oncogene. 2008 Dec;27 Suppl 1:S93-104. doi: 10.1038/onc.2009.47. Oncogene. 2008. PMID: 19641510 Review.
Cited by
-
Cerebral Ischemic Preconditioning Aggravates Death of Oligodendrocytes.Biomolecules. 2022 Dec 14;12(12):1872. doi: 10.3390/biom12121872. Biomolecules. 2022. PMID: 36551300 Free PMC article.
-
Attenuation of Glutamate-Induced Excitotoxicity by Withanolide-A in Neuron-Like Cells: Role for PI3K/Akt/MAPK Signaling Pathway.Mol Neurobiol. 2018 Apr;55(4):2725-2739. doi: 10.1007/s12035-017-0515-5. Epub 2017 Apr 26. Mol Neurobiol. 2018. PMID: 28447311
-
NF-Y-dependent regulation of glutamate receptor 4 expression and cell survival in cells of the oligodendrocyte lineage.Glia. 2018 Sep;66(9):1896-1914. doi: 10.1002/glia.23446. Epub 2018 Apr 27. Glia. 2018. PMID: 29704264 Free PMC article.
-
Perinatal Brain Injury As a Consequence of Preterm Birth and Intrauterine Inflammation: Designing Targeted Stem Cell Therapies.Front Neurosci. 2017 Apr 10;11:200. doi: 10.3389/fnins.2017.00200. eCollection 2017. Front Neurosci. 2017. PMID: 28442989 Free PMC article. Review.
-
Embracing oligodendrocyte diversity in the context of perinatal injury.Neural Regen Res. 2017 Oct;12(10):1575-1585. doi: 10.4103/1673-5374.217320. Neural Regen Res. 2017. PMID: 29171412 Free PMC article. Review.
References
-
- Alberdi E, Sanchez-Gomez M, Marino A, Matute C. Ca2+ influx through AMPA or kainate receptors alone is sufficient to initiate excitotoxicity in cultured oligodendrocytes. Neurobiol Disease. 2002;9:234–243. - PubMed
-
- Back SA, Craig A, Kayton RJ, Luo NL, Meshul CK, Allcock N, Fern R. Hypoxia-ischemia preferentially triggers glutamate depletion from oligodendroglia and axons in perinatal cerebral white matter. J Cereb Blood Flow Metab. 2007;27:334–347. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
