Intrathecal substance P-saporin in the dog: efficacy in bone cancer pain
- PMID: 24195949
- PMCID: PMC3868346
- DOI: 10.1097/ALN.0b013e3182a95188
Intrathecal substance P-saporin in the dog: efficacy in bone cancer pain
Abstract
Background: Substance P-saporin (SP-SAP), a chemical conjugate of substance P and a recombinant version of the ribosome-inactivating protein, saporin, when administered intrathecally, acts as a targeted neurotoxin producing selective destruction of superficial neurokinin-1 receptor-bearing cells in the spinal dorsal horn. The goal of this study was to provide proof-of-concept data that a single intrathecal injection of SP-SAP could safely provide effective pain relief in spontaneous bone cancer pain in companion (pet) dogs.
Methods: In a single-blind, controlled study, 70 companion dogs with bone cancer pain were randomized to standard-of-care analgesic therapy alone (control, n=35) or intrathecal SP-SAP (20-60 µg) in addition to standard-of-care analgesic therapy (n=35). Activity, pain scores, and videography data were collected at baseline, 2 weeks postrandomization, and then monthly until death.
Results: Although the efficacy results at the 2-week postrandomization point were equivocal, the outcomes evaluated beyond 2 weeks revealed a positive effect of SP-SAP on chronic pain management. Significantly, more dogs in the control group (74%) required unblinding and adjustment in analgesic protocol or euthanasia within 6 weeks of randomization than dogs that were treated with SP-SAP (24%; P<0.001); and overall, dogs in the control group required unblinding significantly sooner than dogs that had been treated with SP-SAP (P<0.01).
Conclusion: Intrathecal administration of SP-SAP in dogs with bone cancer produces a time-dependent antinociceptive effect with no evidence of development of deafferentation pain syndrome which can be seen with neurolytic therapies.
Conflict of interest statement
The authors declare no competing interests.
Figures
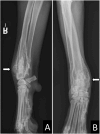

Comment in
-
Substance P-saporin for bone cancer pain in dogs: can man's best friend solve the lost in translation problem in analgesic development?Anesthesiology. 2013 Nov;119(5):999-1000. doi: 10.1097/ALN.0b013e3182a951a2. Anesthesiology. 2013. PMID: 24195943 Free PMC article. No abstract available.
References
-
- Honore P, Mantyh PW. Bone cancer pain: From mechanism to model to therapy. Pain Med. 2000;1:303–3. - PubMed
-
- Brescia FJ, Portenoy RK, Ryan M, Krasnoff L, Gray G. Pain, opioid use, and survival in hospitalized patients with advanced cancer. J Clin Oncol. 1992;10:149–55. - PubMed
-
- Mueller F, Fuchs B, Kaser-Hotz B. Comparative biology of human and canine osteosarcoma. Anticancer Res. 2007;27:155–64. - PubMed
-
- Withrow SJ, Powers BE, Straw RC, Wilkins RM. Comparative aspects of osteosarcoma. Dog versus man. Clin Orthop RelatRes. 1991;270:159–68. - PubMed
-
- MacEwen EG. Spontaneous tumors in dogs and cats: Models for the study of cancer biology and treatment. Cancer Metastasis Rev. 1990;9:125–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

