Advances in nucleophilic phosphine catalysis of alkenes, allenes, alkynes, and MBHADs
- PMID: 24196409
- PMCID: PMC3896345
- DOI: 10.1039/c3cc47368f
Advances in nucleophilic phosphine catalysis of alkenes, allenes, alkynes, and MBHADs
Abstract
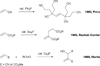
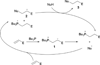
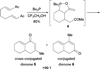
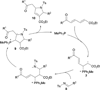
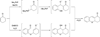
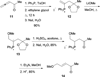
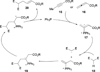
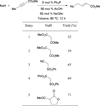
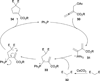
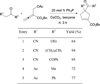
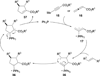
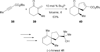
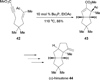
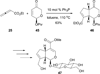
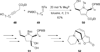
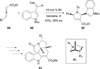
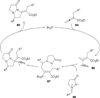
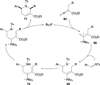
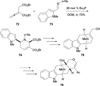
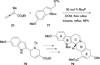
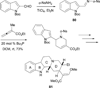
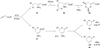
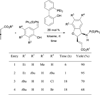
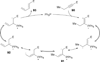
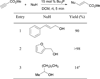
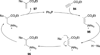
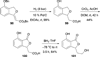
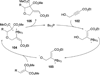
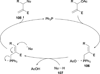
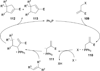
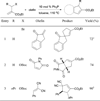
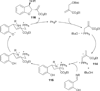
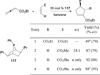
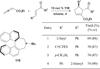
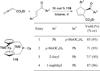
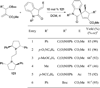
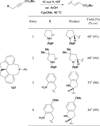
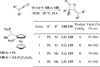
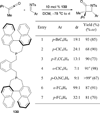
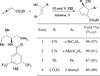
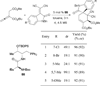
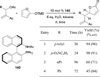
In nucleophilic phosphine catalysis, tertiary phosphines undergo conjugate additions to activated carbon-carbon multiple bonds to form β-phosphonium enolates, β-phosphonium dienolates, β-phosphonium enoates, and vinyl phosphonium ylides as intermediates. When these reactive zwitterionic species react with nucleophiles and electrophiles, they may generate carbo- and heterocycles with multifarious molecular architectures. This article describes the reactivities of these phosphonium zwitterions, the applications of phosphine catalysis in the syntheses of biologically active compounds and natural products, and recent developments in the enantioselective phosphine catalysis.
Figures



















































References
-
- Takashina N, Price CC. J. Am. Chem. Soc. 1962;84:489.
-
- Rauhut MM, Currier H. US 3 074 999, 1963. Chem. Abstr. 1963;58:66109.
-
- Morita K, Suzuki Z, Hirose H. Bull. Chem. Soc. Jpn. 1968;41:2815.
- Baylis AB, Hillman MED. DE 2 155 113, 1972. Chem. Abstr. 1972;77:34174.
-
- White DA, Baizer MM. Tetrahedron Lett. 1973;14:3597.
-
- Stewart IC, Bergman RG, Toste FD. J. Am. Chem. Soc. 2003;125:8696. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

