PAH1-encoded phosphatidate phosphatase plays a role in the growth phase- and inositol-mediated regulation of lipid synthesis in Saccharomyces cerevisiae
- PMID: 24196957
- PMCID: PMC3861629
- DOI: 10.1074/jbc.M113.525766
PAH1-encoded phosphatidate phosphatase plays a role in the growth phase- and inositol-mediated regulation of lipid synthesis in Saccharomyces cerevisiae
Abstract
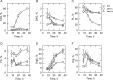
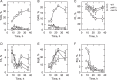
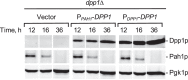
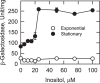
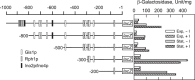
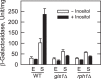
In the yeast Saccharomyces cerevisiae, the synthesis of phospholipids in the exponential phase of growth occurs at the expense of the storage lipid triacylglycerol. As exponential phase cells progress into the stationary phase, the synthesis of triacylglycerol occurs at the expense of phospholipids. Early work indicates a role of the phosphatidate phosphatase (PAP) in this metabolism; the enzyme produces the diacylglycerol needed for the synthesis of triacylglycerol and simultaneously controls the level of phosphatidate for the synthesis of phospholipids. Four genes (APP1, DPP1, LPP1, and PAH1) encode PAP activity in yeast, and it has been unclear which gene is responsible for the synthesis of triacylglycerol throughout growth. An analysis of lipid synthesis and composition, as well as PAP activity in various PAP mutant strains, showed the essential role of PAH1 in triacylglycerol synthesis throughout growth. Pah1p is a phosphorylated enzyme whose in vivo function is dependent on its dephosphorylation by the Nem1p-Spo7p protein phosphatase complex. nem1Δ mutant cells exhibited defects in triacylglycerol synthesis and lipid metabolism that mirrored those imparted by the pah1Δ mutation, substantiating the importance of Pah1p dephosphorylation throughout growth. An analysis of cells bearing PPAH1-lacZ and PPAH1-DPP1 reporter genes showed that PAH1 expression was induced throughout growth and that the induction in the stationary phase was stimulated by inositol supplementation. A mutant analysis indicated that the Ino2p/Ino4p/Opi1p regulatory circuit and transcription factors Gis1p and Rph1p mediated this regulation.
Keywords: Diacylglycerol; Lipids; Phosphatase; Phosphatidate; Phospholipid; Phospholipid Metabolism; Triacylglycerol; Yeast.
Figures



Similar articles
-
Yeast PAH1-encoded phosphatidate phosphatase controls the expression of CHO1-encoded phosphatidylserine synthase for membrane phospholipid synthesis.J Biol Chem. 2017 Aug 11;292(32):13230-13242. doi: 10.1074/jbc.M117.801720. Epub 2017 Jul 3. J Biol Chem. 2017. PMID: 28673963 Free PMC article.
-
Phosphatidate phosphatase, a key regulator of lipid homeostasis.Biochim Biophys Acta. 2013 Mar;1831(3):514-22. doi: 10.1016/j.bbalip.2012.08.006. Epub 2012 Aug 14. Biochim Biophys Acta. 2013. PMID: 22910056 Free PMC article. Review.
-
Phosphorylation of phosphatidate phosphatase regulates its membrane association and physiological functions in Saccharomyces cerevisiae: identification of SER(602), THR(723), AND SER(744) as the sites phosphorylated by CDC28 (CDK1)-encoded cyclin-dependent kinase.J Biol Chem. 2011 Jan 14;286(2):1486-98. doi: 10.1074/jbc.M110.155598. Epub 2010 Nov 16. J Biol Chem. 2011. PMID: 21081492 Free PMC article.
-
Discoveries of the phosphatidate phosphatase genes in yeast published in the Journal of Biological Chemistry.J Biol Chem. 2019 Feb 1;294(5):1681-1689. doi: 10.1074/jbc.TM118.004159. Epub 2018 Jul 30. J Biol Chem. 2019. PMID: 30061152 Free PMC article. Review.
-
The Saccharomyces cerevisiae actin patch protein App1p is a phosphatidate phosphatase enzyme.J Biol Chem. 2012 Nov 23;287(48):40186-96. doi: 10.1074/jbc.M112.421776. Epub 2012 Nov 8. J Biol Chem. 2012. PMID: 23071111 Free PMC article.
Cited by
-
Uncovering the Role of the Yeast Lysine Acetyltransferase NuA4 in the Regulation of Nuclear Shape and Lipid Metabolism.Mol Cell Biol. 2024;44(7):273-288. doi: 10.1080/10985549.2024.2366206. Epub 2024 Jul 4. Mol Cell Biol. 2024. PMID: 38961766 Free PMC article.
-
Altered Lipid Synthesis by Lack of Yeast Pah1 Phosphatidate Phosphatase Reduces Chronological Life Span.J Biol Chem. 2015 Oct 16;290(42):25382-94. doi: 10.1074/jbc.M115.680314. Epub 2015 Sep 3. J Biol Chem. 2015. PMID: 26338708 Free PMC article.
-
Active site determinants of yeast Pah1 phosphatidate phosphatase activity and cellular functions.J Biol Chem. 2025 Aug;301(8):110492. doi: 10.1016/j.jbc.2025.110492. Epub 2025 Jul 17. J Biol Chem. 2025. PMID: 40680843 Free PMC article.
-
Phosphorylation regulates the ubiquitin-independent degradation of yeast Pah1 phosphatidate phosphatase by the 20S proteasome.J Biol Chem. 2015 May 1;290(18):11467-78. doi: 10.1074/jbc.M115.648659. Epub 2015 Mar 25. J Biol Chem. 2015. PMID: 25809482 Free PMC article.
-
Vitamin B6, B12 and folate modulate deregulated pathways and protein aggregation in yeast model of Huntington disease.3 Biotech. 2023 Mar;13(3):96. doi: 10.1007/s13205-023-03525-y. Epub 2023 Feb 24. 3 Biotech. 2023. PMID: 36852176 Free PMC article.
References
-
- Smith S. W., Weiss S. B., Kennedy E. P. (1957) The enzymatic dephosphorylation of phosphatidic acids. J. Biol. Chem. 228, 915–922 - PubMed
-
- Kennedy E. P., Weiss S. B. (1956) The function of cytidine coenzyme in the biosynthesis of phospholipids. J. Biol. Chem. 222, 193–214 - PubMed
-
- Borkenhagen L. F., Kennedy E. P. (1957) The enzymatic synthesis of cytidine diphosphate choline. J. Biol. Chem. 227, 951–962 - PubMed
-
- Weiss S. B., Smith S. W., Kennedy E. P. (1958) The enzymatic formation of lecithin from cytidine diphosphate choline and d-1,2-diglyceride. J. Biol. Chem. 231, 53–64 - PubMed
-
- Kennedy E. P. (1956) The synthesis of cytidine diphosphate choline, cytidine diphosphate ethanolamine, and related compounds. J. Biol. Chem. 222, 185–191 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

