Activation of the FGFR-STAT3 pathway in breast cancer cells induces a hyaluronan-rich microenvironment that licenses tumor formation
- PMID: 24197137
- PMCID: PMC3933218
- DOI: 10.1158/0008-5472.CAN-13-2469
Activation of the FGFR-STAT3 pathway in breast cancer cells induces a hyaluronan-rich microenvironment that licenses tumor formation
Abstract
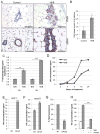
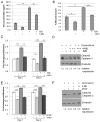
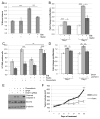
Aberrant activation of fibroblast growth factor receptors (FGFR) contributes to breast cancer growth, progression, and therapeutic resistance. Because of the complex nature of the FGF/FGFR axis, and the numerous effects of FGFR activation on tumor cells and the surrounding microenvironment, the specific mechanisms through which aberrant FGFR activity contributes to breast cancer are not completely understood. We show here that FGFR activation induces accumulation of hyaluronan within the extracellular matrix and that blocking hyaluronan synthesis decreases proliferation, migration, and therapeutic resistance. Furthermore, FGFR-mediated hyaluronan accumulation requires activation of the STAT3 pathway, which regulates expression of hyaluronan synthase 2 (HAS2) and subsequent hyaluronan synthesis. Using a novel in vivo model of FGFR-dependent tumor growth, we demonstrate that STAT3 inhibition decreases both FGFR-driven tumor growth and hyaluronan levels within the tumor. Finally, our results suggest that combinatorial therapies inhibiting both FGFR activity and hyaluronan synthesis is more effective than targeting either pathway alone and may be a relevant therapeutic approach for breast cancers associated with high levels of FGFR activity. In conclusion, these studies indicate a novel targetable mechanism through which FGFR activation in breast cancer cells induces a protumorigenic microenvironment.
Conflict of interest statement
Figures




References
-
- Schwertfeger KL. Fibroblast growth factors in development and cancer: insights from the mammary and prostate glands. Curr Drug Targets. 2009;10:632–44. - PubMed
-
- Dieci MV, Arnedos M, Andre F, Soria JC. Fibroblast growth factor receptor inhibitors as a cancer treatment: from a biologic rationale to medical perspectives. Cancer Discov. 2013;3:264–79. - PubMed
-
- Andre F, Bachelot T, Campone M, et al. Targeting FGFR with Dovitinib (TKI258): Preclinical and Clinical Data in Breast Cancer. Clin Cancer Res. 2013;19:3693–702. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

