Three floral volatiles contribute to differential pollinator attraction in monkeyflowers (Mimulus)
- PMID: 24198269
- PMCID: PMC3922836
- DOI: 10.1242/jeb.092213
Three floral volatiles contribute to differential pollinator attraction in monkeyflowers (Mimulus)
Abstract
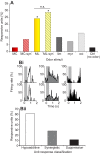
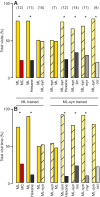
Flowering plants employ a wide variety of signals, including scent, to attract the attention of pollinators. In this study we investigated the role of floral scent in mediating differential attraction between two species of monkeyflowers (Mimulus) reproductively isolated by pollinator preference. The emission rate and chemical identity of floral volatiles differ between the bumblebee-pollinated Mimulus lewisii and the hummingbird-pollinated M. cardinalis. Mimulus lewisii flowers produce an array of volatiles dominated by d-limonene, β-myrcene and E-β-ocimene. Of these three monoterpenes, M. cardinalis flowers produce only d-limonene, released at just 0.9% the rate of M. lewisii flowers. Using the Bombus vosnesenskii bumblebee, an important pollinator of M. lewisii, we conducted simultaneous gas chromatography with extracellular recordings in the bumblebee antennal lobe. Results from these experiments revealed that these three monoterpenes evoke significant neural responses, and that a synthetic mixture of the three volatiles evokes the same responses as the natural scent. Furthermore, the neural population shows enhanced responses to the M. lewisii scent over the scent of M. cardinalis. This neural response is reflected in behavior; in two-choice assays, bumblebees investigate artificial flowers scented with M. lewisii more frequently than ones scented with M. cardinalis, and in synthetic mixtures the three monoterpenes are necessary and sufficient to recapitulate responses to the natural scent of M. lewisii. In this system, floral scent alone is sufficient to elicit differential visitation by bumblebees, implying a strong role of scent in the maintenance of reproductive isolation between M. lewisii and M. cardinalis.
Keywords: Antennal lobe; Floral scent; Insect behavior; Olfaction; Speciation; Terpene.
Figures




Similar articles
-
Floral volatile alleles can contribute to pollinator-mediated reproductive isolation in monkeyflowers (Mimulus).Plant J. 2014 Dec;80(6):1031-42. doi: 10.1111/tpj.12702. Epub 2014 Nov 7. Plant J. 2014. PMID: 25319242 Free PMC article.
-
"Hummingbird" floral traits interact synergistically to discourage visitation by bumble bee foragers.Ecology. 2017 Feb;98(2):489-499. doi: 10.1002/ecy.1661. Epub 2017 Jan 9. Ecology. 2017. PMID: 27864943
-
Ancient and recent introgression shape the evolutionary history of pollinator adaptation and speciation in a model monkeyflower radiation (Mimulus section Erythranthe).PLoS Genet. 2021 Feb 22;17(2):e1009095. doi: 10.1371/journal.pgen.1009095. eCollection 2021 Feb. PLoS Genet. 2021. PMID: 33617525 Free PMC article.
-
Floral isolation, specialized pollination, and pollinator behavior in orchids.Annu Rev Entomol. 2009;54:425-46. doi: 10.1146/annurev.ento.54.110807.090603. Annu Rev Entomol. 2009. PMID: 19067636 Review.
-
β-Ocimene, a Key Floral and Foliar Volatile Involved in Multiple Interactions between Plants and Other Organisms.Molecules. 2017 Jul 13;22(7):1148. doi: 10.3390/molecules22071148. Molecules. 2017. PMID: 28703755 Free PMC article. Review.
Cited by
-
Glomerular Organization in the Antennal Lobe of the Oriental Fruit Fly Bactrocera dorsalis.Front Neuroanat. 2018 Aug 29;12:71. doi: 10.3389/fnana.2018.00071. eCollection 2018. Front Neuroanat. 2018. PMID: 30233333 Free PMC article.
-
Terpenes and Terpenoids in Plants: Interactions with Environment and Insects.Int J Mol Sci. 2020 Oct 6;21(19):7382. doi: 10.3390/ijms21197382. Int J Mol Sci. 2020. PMID: 33036280 Free PMC article. Review.
-
Male flowers of Aconitum compensate for toxic pollen with increased floral signals and rewards for pollinators.Sci Rep. 2019 Nov 11;9(1):16498. doi: 10.1038/s41598-019-53355-3. Sci Rep. 2019. PMID: 31712605 Free PMC article.
-
Transcriptomic analysis of deceptively pollinated Arum maculatum (Araceae) reveals association between terpene synthase expression in floral trap chamber and species-specific pollinator attraction.G3 (Bethesda). 2022 Aug 25;12(9):jkac175. doi: 10.1093/g3journal/jkac175. G3 (Bethesda). 2022. PMID: 35861391 Free PMC article.
-
Extreme diversification of floral volatiles within and among species of Lithophragma (Saxifragaceae).Proc Natl Acad Sci U S A. 2019 Mar 5;116(10):4406-4415. doi: 10.1073/pnas.1809007116. Epub 2019 Feb 14. Proc Natl Acad Sci U S A. 2019. PMID: 30765532 Free PMC article.
References
-
- Alarcón R., Waser N. M., Ollerton J. (2008). Year-to-year variation in the topology of a plant pollinator interaction network. Oikos 117, 1796-1807
-
- Aldridge G., Campbell D. R. (2007). Variation in pollinator preference between two Ipomopsis contact sites that differ in hybridization rate. Evolution 61, 99-110 - PubMed
-
- Ayasse M., Schiestl F. P., Paulus H. F., Löfstedt C., Hansson B., Ibarra F., Francke W. (2000). Evolution of reproductive strategies in the sexually deceptive orchid Ophrys sphegodes: how does flower-specific variation of odor signals influence reproductive success? Evolution 54, 1995-2006 - PubMed
-
- Beardsley P. M., Yen A., Olmstead R. G. (2003). AFLP phylogeny of Mimulus section Erythranthe and the evolution of hummingbird pollination. Evolution 57, 1397-1410 - PubMed
-
- Bradshaw H. D., Jr, Schemske D. W. (2003). Allele substitution at a flower colour locus produces a pollinator shift in monkeyflowers. Nature 426, 176-178 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

