Modality-based organization of ascending somatosensory axons in the direct dorsal column pathway
- PMID: 24198362
- PMCID: PMC3818546
- DOI: 10.1523/JNEUROSCI.3429-13.2013
Modality-based organization of ascending somatosensory axons in the direct dorsal column pathway
Abstract
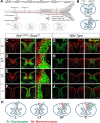
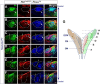
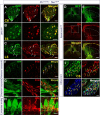
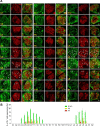
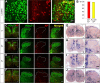
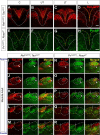
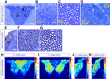
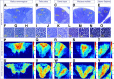
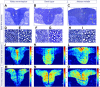
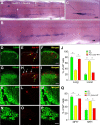
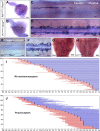
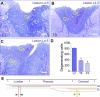
The long-standing doctrine regarding the functional organization of the direct dorsal column (DDC) pathway is the "somatotopic map" model, which suggests that somatosensory afferents are primarily organized by receptive field instead of modality. Using modality-specific genetic tracing, here we show that ascending mechanosensory and proprioceptive axons, two main types of the DDC afferents, are largely segregated into a medial-lateral pattern in the mouse dorsal column and medulla. In addition, we found that this modality-based organization is likely to be conserved in other mammalian species, including human. Furthermore, we identified key morphological differences between these two types of afferents, which explains how modality segregation is formed and why a rough "somatotopic map" was previously detected. Collectively, our results establish a new functional organization model for the mammalian direct dorsal column pathway and provide insight into how somatotopic and modality-based organization coexist in the central somatosensory pathway.
Figures



References
-
- Brown AG, editor. Organization in the spinal cord. New York: Springer; 1981.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
