Neural encoding and integration of learned probabilistic sequences in avian sensory-motor circuitry
- PMID: 24198363
- PMCID: PMC3818547
- DOI: 10.1523/JNEUROSCI.2181-13.2013
Neural encoding and integration of learned probabilistic sequences in avian sensory-motor circuitry
Abstract
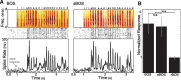
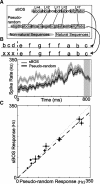
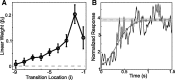
Many complex behaviors, such as human speech and birdsong, reflect a set of categorical actions that can be flexibly organized into variable sequences. However, little is known about how the brain encodes the probabilities of such sequences. Behavioral sequences are typically characterized by the probability of transitioning from a given action to any subsequent action (which we term "divergence probability"). In contrast, we hypothesized that neural circuits might encode the probability of transitioning to a given action from any preceding action (which we term "convergence probability"). The convergence probability of repeatedly experienced sequences could naturally become encoded by Hebbian plasticity operating on the patterns of neural activity associated with those sequences. To determine whether convergence probability is encoded in the nervous system, we investigated how auditory-motor neurons in vocal premotor nucleus HVC of songbirds encode different probabilistic characterizations of produced syllable sequences. We recorded responses to auditory playback of pseudorandomly sequenced syllables from the bird's repertoire, and found that variations in responses to a given syllable could be explained by a positive linear dependence on the convergence probability of preceding sequences. Furthermore, convergence probability accounted for more response variation than other probabilistic characterizations, including divergence probability. Finally, we found that responses integrated over >7-10 syllables (∼700-1000 ms) with the sign, gain, and temporal extent of integration depending on convergence probability. Our results demonstrate that convergence probability is encoded in sensory-motor circuitry of the song-system, and suggest that encoding of convergence probability is a general feature of sensory-motor circuits.
Figures




References
-
- Barlow HB. Possible principles underlying the transformation of sensory messages. In: Rosenblith WA, editor. Sensory communication. Cambridge, MA: MIT; 1961. pp. 217–234.
-
- Bouchard KE, Ganguly S, Brainard MS. Hebbian mechanisms underlying the learning of Markov sequence probabilities. Soc Neurosci Abstr. 2012;38:846.16.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
