Genetic dissection of retinal inputs to brainstem nuclei controlling image stabilization
- PMID: 24198370
- PMCID: PMC3818553
- DOI: 10.1523/JNEUROSCI.2778-13.2013
Genetic dissection of retinal inputs to brainstem nuclei controlling image stabilization
Abstract
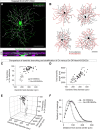
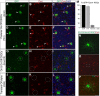
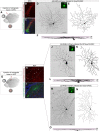
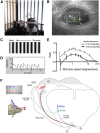
When the head rotates, the image of the visual world slips across the retina. A dedicated set of retinal ganglion cells (RGCs) and brainstem visual nuclei termed the "accessory optic system" (AOS) generate slip-compensating eye movements that stabilize visual images on the retina and improve visual performance. Which types of RGCs project to each of the various AOS nuclei remain unresolved. Here we report a new transgenic mouse line, Hoxd10-GFP, in which the RGCs projecting to all the AOS nuclei are fluorescently labeled. Electrophysiological recordings of Hoxd10-GFP RGCs revealed that they include all three subtypes of On direction-selective RGCs (On-DSGCs), responding to upward, downward, or forward motion. Hoxd10-GFP RGCs also include one subtype of On-Off DSGCs tuned for forward motion. Retrograde circuit mapping with modified rabies viruses revealed that the On-DSGCs project to the brainstem centers involved in both horizontal and vertical retinal slip compensation. In contrast, the On-Off DSGCs labeled in Hoxd10-GFP mice projected to AOS nuclei controlling horizontal but not vertical image stabilization. Moreover, the forward tuned On-Off DSGCs appear physiologically and molecularly distinct from all previously genetically identified On-Off DSGCs. These data begin to clarify the cell types and circuits underlying image stabilization during self-motion, and they support an unexpected diversity of DSGC subtypes.
Figures








Similar articles
-
Molecular Fingerprinting of On-Off Direction-Selective Retinal Ganglion Cells Across Species and Relevance to Primate Visual Circuits.J Neurosci. 2019 Jan 2;39(1):78-95. doi: 10.1523/JNEUROSCI.1784-18.2018. Epub 2018 Oct 30. J Neurosci. 2019. PMID: 30377226 Free PMC article.
-
Transgenic mice reveal unexpected diversity of on-off direction-selective retinal ganglion cell subtypes and brain structures involved in motion processing.J Neurosci. 2011 Jun 15;31(24):8760-9. doi: 10.1523/JNEUROSCI.0564-11.2011. J Neurosci. 2011. PMID: 21677160 Free PMC article.
-
Identification of retinal ganglion cells and their projections involved in central transmission of information about upward and downward image motion.PLoS One. 2009;4(1):e4320. doi: 10.1371/journal.pone.0004320. Epub 2009 Jan 29. PLoS One. 2009. PMID: 19177171 Free PMC article.
-
Direction selectivity in the retina: symmetry and asymmetry in structure and function.Nat Rev Neurosci. 2012 Feb 8;13(3):194-208. doi: 10.1038/nrn3165. Nat Rev Neurosci. 2012. PMID: 22314444 Review.
-
New directions in retinal research.Trends Neurosci. 2003 Jul;26(7):379-85. doi: 10.1016/S0166-2236(03)00167-X. Trends Neurosci. 2003. PMID: 12850434 Review.
Cited by
-
Adaptive Acceleration of Visually Evoked Smooth Eye Movements in Mice.J Neurosci. 2016 Jun 22;36(25):6836-49. doi: 10.1523/JNEUROSCI.0067-16.2016. J Neurosci. 2016. PMID: 27335412 Free PMC article.
-
An ON-type direction-selective ganglion cell in primate retina.Nature. 2023 Nov;623(7986):381-386. doi: 10.1038/s41586-023-06659-4. Epub 2023 Oct 25. Nature. 2023. PMID: 37880369 Free PMC article.
-
Tbr2 is required to generate a neural circuit mediating the pupillary light reflex.J Neurosci. 2014 Apr 16;34(16):5447-53. doi: 10.1523/JNEUROSCI.0035-14.2014. J Neurosci. 2014. PMID: 24741035 Free PMC article.
-
The dynamic receptive fields of retinal ganglion cells.Prog Retin Eye Res. 2018 Nov;67:102-117. doi: 10.1016/j.preteyeres.2018.06.003. Epub 2018 Jun 23. Prog Retin Eye Res. 2018. PMID: 29944919 Free PMC article. Review.
-
Congenital Nystagmus Gene FRMD7 Is Necessary for Establishing a Neuronal Circuit Asymmetry for Direction Selectivity.Neuron. 2016 Jan 6;89(1):177-93. doi: 10.1016/j.neuron.2015.11.032. Epub 2015 Dec 17. Neuron. 2016. PMID: 26711119 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
