Human immunodeficiency virus-1 Tat protein increases the number of inhibitory synapses between hippocampal neurons in culture
- PMID: 24198379
- PMCID: PMC3818559
- DOI: 10.1523/JNEUROSCI.1312-13.2013
Human immunodeficiency virus-1 Tat protein increases the number of inhibitory synapses between hippocampal neurons in culture
Abstract
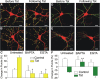
Synaptodendritic damage correlates with cognitive decline in many neurodegenerative diseases, including human immunodeficiency virus-1 (HIV-1)-associated neurocognitive disorders (HAND). Because HIV-1 does not infect neurons, viral-mediated toxicity is indirect, resulting from released neurotoxins such as the HIV-1 protein transactivator of transcription (Tat). We compared the effects of Tat on inhibitory and excitatory synaptic connections between rat hippocampal neurons using an imaging-based assay that quantified clusters of the scaffolding proteins gephyrin or PSD95 fused to GFP. Tat (24 h) increased the number of GFP-gephyrin puncta and decreased the number of PSD95-GFP puncta. The effects of Tat on inhibitory and excitatory synapse number were mediated via the low-density lipoprotein receptor-related protein and subsequent Ca(2+) influx through GluN2A-containing NMDA receptors (NMDARs). The effects of Tat on synapse number required cell-autonomous activation of Ca(2+)/calmodulin-dependent protein kinase II (CaMKII). Ca(2+) buffering experiments suggested that loss of excitatory synapses required activation of CaMKII in close apposition to the NMDAR, whereas the increase in inhibitory synapses required Ca(2+) diffusion to a more distal site. The increase in inhibitory synapses was prevented by inhibiting the insertion of GABAA receptors into the membrane. Synaptic changes induced by Tat (16 h) were reversed by blocking either GluN2B-containing NMDARs or neuronal nitric oxide synthase, indicating changing roles for pathways activated by NMDAR subtypes during the neurotoxic process. Compensatory changes in the number of inhibitory and excitatory synapses may serve as a novel mechanism to reduce network excitability in the presence of HIV-1 neurotoxins; these changes may inform the development of treatments for HAND.
Figures









References
-
- Avenet P, Léonardon J, Besnard F, Graham D, Frost J, Depoortere H, Langer SZ, Scatton B. Antagonist properties of the sterioisomers of ifenprodil at NR1A/NR2A and NR1A/NR2B subtypes of the NMDA receptor expressed in Xenopus oocytes. Eur J Pharmacol. 1996;296:209–213. doi: 10.1016/0014-2999(95)00700-8. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
