Consciousness in humans and non-human animals: recent advances and future directions
- PMID: 24198791
- PMCID: PMC3814086
- DOI: 10.3389/fpsyg.2013.00625
Consciousness in humans and non-human animals: recent advances and future directions
Abstract
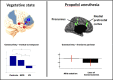
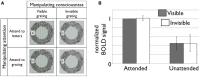
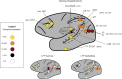
This joint article reflects the authors' personal views regarding noteworthy advances in the neuroscience of consciousness in the last 10 years, and suggests what we feel may be promising future directions. It is based on a small conference at the Samoset Resort in Rockport, Maine, USA, in July of 2012, organized by the Mind Science Foundation of San Antonio, Texas. Here, we summarize recent advances in our understanding of subjectivity in humans and other animals, including empirical, applied, technical, and conceptual insights. These include the evidence for the importance of fronto-parietal connectivity and of "top-down" processes, both of which enable information to travel across distant cortical areas effectively, as well as numerous dissociations between consciousness and cognitive functions, such as attention, in humans. In addition, we describe the development of mental imagery paradigms, which made it possible to identify covert awareness in non-responsive subjects. Non-human animal consciousness research has also witnessed substantial advances on the specific role of cortical areas and higher order thalamus for consciousness, thanks to important technological enhancements. In addition, much progress has been made in the understanding of non-vertebrate cognition relevant to possible conscious states. Finally, major advances have been made in theories of consciousness, and also in their comparison with the available evidence. Along with reviewing these findings, each author suggests future avenues for research in their field of investigation.
Keywords: animals; biotechnology; consciousness; human cognition; neuroimaging; theoretical neuroscience.
Figures



References
-
- Andersen R. A., Gnadt J. W. (1989). Posterior parietal cortex. Rev. Oculomot. Res. 3, 315–335 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

