Inhibition of Hedgehog signaling sensitizes NSCLC cells to standard therapies through modulation of EMT-regulating miRNAs
- PMID: 24199791
- PMCID: PMC3852827
- DOI: 10.1186/1756-8722-6-77
Inhibition of Hedgehog signaling sensitizes NSCLC cells to standard therapies through modulation of EMT-regulating miRNAs
Abstract
Background: Epidermal growth factor receptor- tyrosine kinase inhibitors (EGFR-TKIs) benefit Non-small cell lung cancer (NSCLC) patients, and an EGFR-TKIi erlotinib, is approved for patients with recurrent NSCLC. However, resistance to erlotinib is a major clinical problem. Earlier we have demonstrated the role of Hedgehog (Hh) signaling in Epithelial-to-Mesenchymal transition (EMT) of NSCLC cells, leading to increased proliferation and invasion. Here, we investigated the role of Hh signaling in erlotinib resistance of TGF-β1-induced NSCLC cells that are reminiscent of EMT cells.
Methods: Hh signaling was inhibited by specific siRNA and by GDC-0449, a small molecule antagonist of G protein coupled receptor smoothened in the Hh pathway. Not all NSCLC patients are likely to benefit from EGFR-TKIs and, therefore, cisplatin was used to further demonstrate a role of inhibition of Hh signaling in sensitization of resistant EMT cells. Specific pre- and anti-miRNA preparations were used to study the mechanistic involvement of miRNAs in drug resistance mechanism.
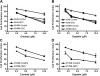
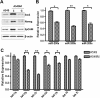
Results: siRNA-mediated inhibition as well as pharmacological inhibition of Hh signaling abrogated resistance of NSCLC cells to erlotinib and cisplatin. It also resulted in re-sensitization of TGF-β1-induced A549 (A549M) cells as well the mesenchymal phenotypic H1299 cells to erlotinib and cisplatin treatment with concomitant up-regulation of cancer stem cell (CSC) markers (Sox2, Nanog and EpCAM) and down-regulation of miR-200 and let-7 family miRNAs. Ectopic up-regulation of miRNAs, especially miR-200b and let-7c, significantly diminished the erlotinib resistance of A549M cells. Inhibition of Hh signaling by GDC-0449 in EMT cells resulted in the attenuation of CSC markers and up-regulation of miR-200b and let-7c, leading to sensitization of EMT cells to drug treatment, thus, confirming a connection between Hh signaling, miRNAs and drug resistance.
Conclusions: We demonstrate that Hh pathway, through EMT-induction, leads to reduced sensitivity to EGFR-TKIs in NSCLCs. Therefore, targeting Hh pathway may lead to the reversal of EMT phenotype and improve the therapeutic efficacy of EGFR-TKIs in NSCLC patients.
Figures
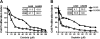
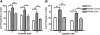
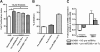
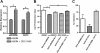
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

