Rapid and reversible responses to IVIG in autoimmune neuromuscular diseases suggest mechanisms of action involving competition with functionally important autoantibodies
- PMID: 24200120
- PMCID: PMC4285221
- DOI: 10.1111/jns5.12048
Rapid and reversible responses to IVIG in autoimmune neuromuscular diseases suggest mechanisms of action involving competition with functionally important autoantibodies
Abstract
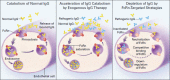
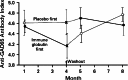
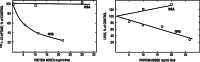
Intravenous immunoglobulin (IVIG) is widely used in autoimmune neuromuscular diseases whose pathogenesis is undefined. Many different effects of IVIG have been demonstrated in vitro, but few studies actually identify the mechanism(s) most important in vivo. Doses and treatment intervals are generally chosen empirically. Recent studies in Guillain-Barré syndrome and chronic inflammatory demyelinating polyneuropathy show that some effects of IVIG are readily reversible and highly dependent on the serum IgG level. This suggests that in some autoantibody-mediated neuromuscular diseases, IVIG directly competes with autoantibodies that reversibly interfere with nerve conduction. Mechanisms of action of IVIG which most likely involve direct competition with autoantibodies include: neutralization of autoantibodies by anti-idiotypes, inhibition of complement deposition, and increasing catabolism of pathologic antibodies by saturating FcRn. Indirect immunomodulatory effects are not as likely to involve competition and may not have the same reversibility and dose-dependency. Pharmacodynamic analyses should be informative regarding most relevant mechanism(s) of action of IVIG as well as the role of autoantibodies in the immunopathogenesis of each disease. Better understanding of the role of autoantibodies and of the target(s) of IVIG could lead to more efficient use of this therapy and better patient outcomes.
Keywords: Guillain-Barré syndrome; anti-idiotypes; autoimmune neuromuscular diseases; chronic inflammatory demyelinating polyneuropathy; intravenous immunoglobulin; multifocal motor neuropathy.
© 2013 The Authors. Journal of the Peripheral Nervous System published by Wiley Periodicals, Inc. on behalf of Peripheral Nerve Society.
Figures
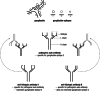

References
-
- Anthony RM, Wermeling F, Ravetch JV. Novel roles for the IgG Fc glycan. Ann N Y Acad Sci. 2012;1253:170–180. - PubMed
-
- Arcila-Londono X, Lewis RA. Guillain-Barre syndrome. Semin Neurol. 2012;32:179–186. - PubMed
-
- Ariga T, Yu RK. Antiglycolipid antibodies in Guillain-Barre syndrome and related diseases: review of clinical features and antibody specificities. J Neurosci Res. 2005;80:1–17. - PubMed
-
- Arsura EL, Bick A, Brunner NG, Namba T, Grob D. High-dose intravenous immunoglobulin in the management of myasthenia gravis. Arch Intern Med. 1986;146:1365–1368. - PubMed
-
- Azulay JP, Blin O, Pouget J, Boucraut J, Bille-Turc F, Carles G, Serratrice G. Intravenous immunoglobulin treatment in patients with motor neuron syndromes associated with anti-GM1 antibodies: a double-blind, placebo-controlled study. Neurology. 1994;44:429–432. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

