ERK as a model for systems biology of enzyme kinetics in cells
- PMID: 24200329
- PMCID: PMC4131290
- DOI: 10.1016/j.cub.2013.09.033
ERK as a model for systems biology of enzyme kinetics in cells
Abstract
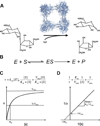
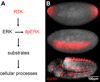
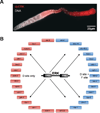
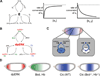
A key step towards a chemical picture of enzyme catalysis was taken in 1913, when Leonor Michaelis and Maud Menten published their studies of sucrose hydrolysis by invertase. Based on a novel experimental design and a mathematical model, their work offered a quantitative view of biochemical kinetics well before the protein nature of enzymes was established and complexes with substrates could be detected. Michaelis-Menten kinetics provides a solid framework for enzyme kinetics in vitro, but what about kinetics in cells, where enzymes can be highly regulated and participate in a multitude of interactions? We discuss this question using the Extracellular Signal Regulated Kinase (ERK), which controls a myriad functions in cells, as a model of an important enzyme for which we have crystal structures, quantitative in vitro assays, and a vast list of binding partners. Despite great progress, we still cannot quantitatively predict how the rates of ERK-dependent reactions respond to genetic and pharmacological perturbations. Achieving this goal, which is important from both fundamental and practical standpoints, requires measuring the rates of enzyme reactions in their native environment and interpreting these measurements using simple but realistic mathematical models--the two elements which served as the cornerstones for Michaelis' and Menten's seminal 1913 paper.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures

References
-
- Michaelis L, Menten ML. Die kinetik der invertinwirkung. Biochem. Z. 1913;49:333–369.
-
- Fruton JS. Proteins, Enzymes, Genes: The Interplay of Chemistry and Biology. Yale University Press; 1999.
-
- Cornish-Bowden A. The origins of enzyme kinetics. FEBS letters. 2013;587(17):2725–2730. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

