Photoactivated rose bengal functionalized chitosan nanoparticles produce antibacterial/biofilm activity and stabilize dentin-collagen
- PMID: 24200522
- PMCID: PMC3966929
- DOI: 10.1016/j.nano.2013.10.010
Photoactivated rose bengal functionalized chitosan nanoparticles produce antibacterial/biofilm activity and stabilize dentin-collagen
Abstract
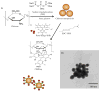
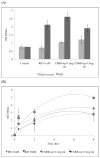
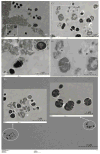
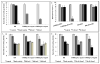
Treatment of infected teeth presents two major challenges: persistence of the bacterial-biofilm within root canals after treatment and compromised structural integrity of the dentin hard-tissue. In this study bioactive polymeric chitosan nanoparticles functionalized with rose-bengal, CSRBnp were developed to produce antibiofilm effects as well as stabilize structural-integrity by photocrosslinking dentin-collagen. CSRBnp were less toxic to fibroblasts and had significant antibacterial activity even in the presence of bovine serum albumin. CSRBnp exerted antibacterial mechanism by adhering to bacterial cell surface, permeabilizing the membrane and lysing the cells subsequent to photodynamic treatment. Photoactivated CSRBnp resulted in reduced viability of Enterococcus faecalis biofilms and disruption of biofilm structure. Incorporation of CSRBnp and photocrosslinking significantly improved resistance to degradation and mechanical strength of dentin-collagen (P<0.05). The functionalized chitosan nanoparticles provided a single-step treatment of infected root dentin by combining the properties of chitosan and that of photosensitizer to eliminate bacterial-biofilms and stabilize dentin-matrix.
From the clinical editor: In this study, bioactive polymeric chitosan nanoparticles functionalized with rose-bengal (a photosensitizer), CSRBnp were developed to produce antibiofilm effects as well as stabilize structural-integrity of dental root dentin by photocrosslinking dentin-collagen, leading to efficient elimination of bacterial-biofilms and stabilization of dentin-matrix.
Keywords: Biofilms; Chitosan; Collagen; Dentin; Functionalized nanoparticles; Photodynamic therapy.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures



References
-
- Lumley PJ, Lucarotti PS, Burke FJ. Ten-year outcome of root fillings in the General Dental Services in England and Wales. Int Endod J. 2008;41:577–85. - PubMed
-
- Nair PN. Pathogenesis of apical periodontitis and the causes of endodontic failures. Crit Rev Oral Biol Med. 2004;15:348–81. - PubMed
-
- Vier FV, Figueiredo JA. Internal apical resorption and its correlation with the type of apical lesion. Int Endod J. 2004;37:730–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

