Molecular architecture of a eukaryotic translational initiation complex
- PMID: 24200810
- PMCID: PMC3836175
- DOI: 10.1126/science.1240585
Molecular architecture of a eukaryotic translational initiation complex
Abstract
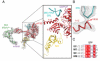
The last step in eukaryotic translational initiation involves the joining of the large and small subunits of the ribosome, with initiator transfer RNA (Met-tRNA(i)(Met)) positioned over the start codon of messenger RNA in the P site. This step is catalyzed by initiation factor eIF5B. We used recent advances in cryo-electron microscopy (cryo-EM) to determine a structure of the eIF5B initiation complex to 6.6 angstrom resolution from <3% of the population, comprising just 5143 particles. The structure reveals conformational changes in eIF5B, initiator tRNA, and the ribosome that provide insights into the role of eIF5B in translational initiation. The relatively high resolution obtained from such a small fraction of a heterogeneous sample suggests a general approach for characterizing the structure of other dynamic or transient biological complexes.
Figures




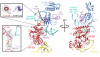
References
-
- Marintchev A, Wagner G. Translation initiation: structures, mechanisms and evolution. Q Rev Biophys. 2004;37:197. - PubMed
-
- Aitken CE, Lorsch JR. A mechanistic overview of translation initiation in eukaryotes. Nat Struct Mol Biol. 2012;19:568. - PubMed
-
- Roll-Mecak A, Shin BS, Dever TE, Burley SK. Engaging the ribosome: universal IFs of translation. Trends Biochem Sci. 2001;26:705. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

