Effects of cyclic AMP response element binding protein-Zhangfei (CREBZF) on the unfolded protein response and cell growth are exerted through the tumor suppressor p53
- PMID: 24200963
- PMCID: PMC3906244
- DOI: 10.4161/cc.27053
Effects of cyclic AMP response element binding protein-Zhangfei (CREBZF) on the unfolded protein response and cell growth are exerted through the tumor suppressor p53
Abstract
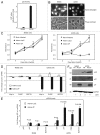
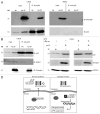
Zhangfei/CREBZF, a basic region-leucine zipper (bLZip) transcription factor, is a potent suppressor of growth and the unfolded protein response (UPR) in some cancer cell lines, including the canine osteosarcoma cell line, D-17. However, the effects of Zhangfei are not universal, and it has no obvious effects on untransformed cells and some cancer cell lines, suggesting that Zhangfei may act through an intermediary that is either not induced or is defective in cells that it does not affect. Here we identify the tumor suppressor protein p53 as this intermediary. We show the following: in cells ectopically expressing Zhangfei, the protein stabilizes p53 and co-localizes with it in cellular nuclei; the bLZip domain of Zhangfei is required for its profound effects on cell growth and interaction with p53. Suppression of p53 by siRNA at least partially inhibits the effects of Zhangfei on the UPR and cell growth. The effects of Zhangfei on D-17 cells is mirrored by its effects on the p53-expressing human osteosarcoma cell line U2OS, while Zhangfei has no effect on the p53-null osteosarcoma cell line MG63. In U2OS cells, Zhangfei displaces the E3 ubiquitin ligase mouse double minute homolog 2 (Mdm2) from its association with p53, suggesting a mechanism for the effects of Zhangfei on p53.
Keywords: Mdm2; Zhangfei/CREBZF; basic-leucine zipper domain; cell cycle; osteosarcoma; p53; protein domains; protein translocation; unfolded protein response.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
