iRFP is a sensitive marker for cell number and tumor growth in high-throughput systems
- PMID: 24200967
- PMCID: PMC3906239
- DOI: 10.4161/cc.26985
iRFP is a sensitive marker for cell number and tumor growth in high-throughput systems
Abstract
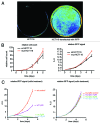
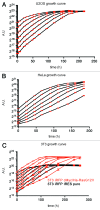
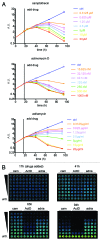
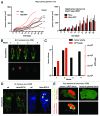
GFP and luciferase are used extensively as markers both in vitro and in vivo although both have limitations. The utility of GFP fluorescence is restricted by high background signal and poor tissue penetrance. Luciferase throughput is limited in vitro by the requirement for cell lysis, while in vivo, luciferase readout is complicated by the need for substrate injection and the dependence on endogenous ATP. Here we show that near-infrared fluorescent protein in combination with widely available near-infrared scanners overcomes these obstacles and allows for the accurate determination of cell number in vitro and tumor growth in vivo in a high-throughput manner and at negligible per-well costs. This system represents a significant advance in tracking cell proliferation in tissue culture as well as in animals, with widespread applications in cell biology.
Keywords: cancer; iRFP cell number quantification; in vivo; near-infrared fluorescence.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
