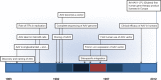
Birth of a new therapeutic platform: 47 years of adeno-associated virus biology from virus discovery to licensed gene therapy
- PMID: 24201212
- PMCID: PMC3831048
- DOI: 10.1038/mt.2013.226
Birth of a new therapeutic platform: 47 years of adeno-associated virus biology from virus discovery to licensed gene therapy
Figures

Similar articles
-
Retinal gene therapy using adeno-associated viral vectors: multiple applications for a small virus.Hum Gene Ther. 2014 Aug;25(8):671-8. doi: 10.1089/hum.2014.2530. Hum Gene Ther. 2014. PMID: 25136913 Free PMC article. No abstract available.
-
My Pathway to Adeno-Associated Virus and Adeno-Associated Virus Gene Therapy: A Personal Perspective.Hum Gene Ther. 2020 May;31(9-10):494-498. doi: 10.1089/hum.2020.29120.bca. Epub 2020 Apr 9. Hum Gene Ther. 2020. PMID: 32275185 Free PMC article. No abstract available.
-
Recombinant Adeno-Associated Virus Gene Therapy in Light of Luxturna (and Zolgensma and Glybera): Where Are We, and How Did We Get Here?Annu Rev Virol. 2019 Sep 29;6(1):601-621. doi: 10.1146/annurev-virology-092818-015530. Epub 2019 Jul 5. Annu Rev Virol. 2019. PMID: 31283441 Free PMC article.
-
Adeno-associated virus at 50: a golden anniversary of discovery, research, and gene therapy success--a personal perspective.Hum Gene Ther. 2015 May;26(5):257-65. doi: 10.1089/hum.2015.025. Epub 2015 Apr 20. Hum Gene Ther. 2015. PMID: 25807962 Free PMC article. Review.
-
Nonintegrating Gene Therapy Vectors.Hematol Oncol Clin North Am. 2017 Oct;31(5):753-770. doi: 10.1016/j.hoc.2017.06.007. Hematol Oncol Clin North Am. 2017. PMID: 28895845 Review.
Cited by
-
Adenovirus-Mediated Gene Delivery: Potential Applications for Gene and Cell-Based Therapies in the New Era of Personalized Medicine.Genes Dis. 2017 Jun;4(2):43-63. doi: 10.1016/j.gendis.2017.04.001. Epub 2017 Apr 27. Genes Dis. 2017. PMID: 28944281 Free PMC article.
-
Development of a Beta Cell-Specific Expression Control Element for Recombinant Adeno-Associated Virus.Hum Gene Ther. 2022 Aug;33(15-16):789-800. doi: 10.1089/hum.2021.219. Epub 2022 May 10. Hum Gene Ther. 2022. PMID: 35297680 Free PMC article.
-
In vivo delivery of CRISPR-Cas9 therapeutics: Progress and challenges.Acta Pharm Sin B. 2021 Aug;11(8):2150-2171. doi: 10.1016/j.apsb.2021.05.020. Epub 2021 May 26. Acta Pharm Sin B. 2021. PMID: 34522582 Free PMC article. Review.
-
Gene Therapy for Regenerative Medicine.Pharmaceutics. 2023 Mar 6;15(3):856. doi: 10.3390/pharmaceutics15030856. Pharmaceutics. 2023. PMID: 36986717 Free PMC article. Review.
-
Structural and Biophysical Analysis of Adeno-Associated Virus Serotype 2 Capsid Assembly Variants.J Virol. 2023 Jul 27;97(7):e0177222. doi: 10.1128/jvi.01772-22. Epub 2023 Jun 13. J Virol. 2023. PMID: 37310260 Free PMC article.
References
-
- Burnett JR., and, Hooper AJ. Alipogene tiparvovec, an adeno-associated virus encoding the Ser(447)X variant of the human lipoprotein lipase gene for the treatment of patients with lipoprotein lipase deficiency. Curr Opin Mol Ther . 2009;11:681–691. - PubMed
-
- Blacklow NR, Hoggan MD, Kapikian AZ, Austin JB., and, Rowe WP. Epidemiology of adenovirus-associated virus infection in a nursery population. . Am J Epidemiol. 1968;88:368–378. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

